
In pancreatic ductal adenocarcinoma (PDAC), stromal cells and matrix proteins form a dense physical barrier that not only confines tumor cells but also severely restricts the penetration of therapeutic agents and CD8+ T lymphocytes. Concurrently, the hyperactivated TGF-β/SMAD signaling pathway further promotes stromal hyperplasia and immunosuppression. Therefore, a drug delivery strategy that enables stromal barrier penetration while preserving stromal integrity, achieves intratumoral drug release, remodels the stroma, and activates the immune system holds significant promise. In this study, we developed enamine N-oxide-modified nanoparticles co-loaded with a stearic acid-conjugated gemcitabine prodrug (GemC18) and the pSMAD2/3 inhibitor galunisertib. The enamine N-oxide on the nanoparticle surface triggers transcytosis. Subsequently, under hypoxic and acidic tumor microenvironments, the nanoparticles undergo charge reversal (neutral to positive), enhancing their penetration capacity. The released galunisertib inhibits TGF-β/SMAD signaling to remodel the stroma, activate antitumor immunity, and synergize with gemcitabine (Gem) for tumor cell elimination. These findings were published in Nano Letters under the title Transcytosis-Triggering Nanoparticles for Overcoming Stromal Barriers and Reversing Immunosuppression in Pancreatic Cancer Combinatorial Therapy (DOI: 10.1021/acs.nanolett.4c06372).
PDAC is one of the most lethal malignancies, with stroma constituting ~90% of the tumor volume. This dense stroma encases tumor cells, forming a physical barrier. Current clinical chemotherapy employs albumin-bound paclitaxel to deplete the stroma, thereby increasing intratumoral Gem concentration. However, this approach inadvertently promotes tumor metastasis, offering only marginal survival benefits (8.5 vs. 6.7 months). Thus, an optimal strategy should preserve stromal integrity while developing drug delivery platforms capable of traversing the stromal barrier to achieve both enhanced intratumoral drug accumulation and microenvironment modulation.
Recent studies highlight transcytosis—a cell-mediated transport mechanism—as a promising approach to overcome PDAC stromal barriers. N-oxide-modified zwitterionic polymers facilitate Adsorption-Mediated Transcytosis (AMT), enabling deep tumor penetration. Consequently, developing N-oxide-based transcytosis-triggering drug delivery systems provides a rational solution for bypassing PDAC stromal barriers.
The PDAC stroma comprises cellular components (e.g., cancer-associated fibroblasts [CAFs], immune cells, pancreatic stellate cells [PSCs]) and non-cellular components (e.g., collagen, hyaluronic acid, fibronectin). Stromal hyperplasia exacerbates hypoxia, acidosis, and immunosuppression, fostering tumor progression. Within this microenvironment, the TGF-β/SMAD pathway plays a central role. TGF-β/SMAD signaling activates CAFs and PSCs, driving excessive collagen and hyaluronic acid secretion.
1. ECM Deposition: Collagen and fibronectin accumulation compresses tumor vasculature, impairs lymphatic drainage, and generates interstitial pressure equivalent to mean arterial pressure, collectively hindering drug delivery.
2. Hypoxia: Vascular collapse creates an oxygen gradient, inducing hypoxia. Tumor cells counteract reactive oxygen species (ROS) by overexpressing glutathione (GSH), while hypoxia-driven glycolysis produces lactate, acidifying the microenvironment.
3. Immunosuppression: Lactate accumulation inhibits CD8+ T and natural killer cells, promotes immunosuppressive FOXP3+ regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and blocks CD8+ T cell infiltration via physical barriers. TGF-β/SMAD signaling directly induces M2-like tumor-associated macrophage (TAM) polarization, Treg expansion, and CD8+ T cell suppression.
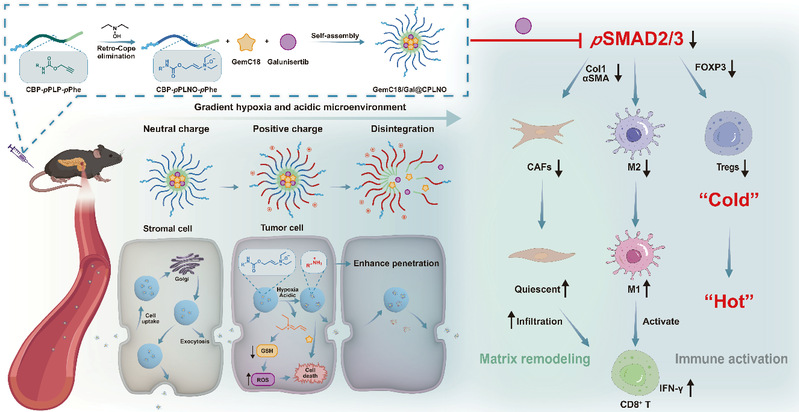
Figure. Design and Mechanism of GemC18/Gal@CPLNO Nanoparticles
As illustrated in Figure 1, the hypoxia/acidic-responsive nanoparticle system (GemC18/Gal@CPLNO) operates via: 1) Transcytosis Initiation: Surface enamine N-oxide triggers AMT to traverse the outer stromal barrier; 2) Microenvironment-Responsive Charge Reversal: Hypoxia: Enamine N-oxide is reduced to tertiary amine derivatives, releasing unsaturated iminium ions that undergo Michael addition with GSH. Acidic pH: Lysine residues on the polymer backbone protonate, switching surface charge from neutral to positive, enhancing penetration via transcytosis. 3) Dual Payload Release: Galunisertib: Inhibits TGF-β/SMAD signaling, suppresses CAF activation, remodels stroma, and reverses immunosuppression. GemC18: Hydrolyzes to Gem, exerting cytotoxic effects. 4) Synergistic Antitumor Effects
Galunisertib remodels the immunosuppressive microenvironment, enhances CD8+ T cell infiltration, and synergizes with Gem to suppress tumor growth.
Authorship
First Author: Shilin Zhang (Master’s candidate, Jiang Chen & Sun Tao Group)
Corresponding Authors: Associate Prof. Tao Sun and Prof. Chen Jiang