
Metastasis is the foremost cause of cancer-related death. Current clinical treatments, including tumor resection, chemotherapy and radiation therapy, can effectively remove the primary tumor but still fail to inhibit and might even induce tumor metastasis.Therefore, exploring an effective metastasis-specific therapeutic based on the underlying mechanism of tumor metastasis is critical to cancer therapy. The progression of metastasis includes three main steps: the invasion, circulation and the colonization phases. (i) In the invasion phase, the tumor cells escape from the primary tumor tissue to surrounding tissues and extravasate into the neighboring blood vessels. In this phase, the motility and invasiveness of tumor cells were enhanced by epithelial–mesenchymal transition (EMT). (ii) In the circulation phase, the tumor cells that enter and survive in the bloodstream are called circulating tumor cells (CTCs). During this phase, platelets are bound to CTCs, forming the special structure called “micro-thrombi”, which protects CTCs from natural killer cells (NK cells) in circulation. (iii) In the colonization phase, CTCs infiltrate and colonize in the distant organs. The distant organs that provide suitable niche for CTCs colonization are defined as the pre-metastatic niche that is an inflammatory and immunosuppressive site. Hence, the therapeutics targeting a single step of metastasis, failed to halt the multi-steps of metastasis.
Recently, Professor Jun Chen from School of Pharmacy, Fudan University, has reported a systemic metastasis-targeted nanotherapeutic, which could reinforce tumor surgical resection and chemotherapy and was used for combating tumor metastasis. The relevant studies were published online in Nature Communications (IF=12.121), entitled “Systemic metastasis-targeted nanotherapeutic reinforces tumor surgical resection and chemotherapy”.
Herein, the researchers designed a systemic metastasis-targeted nanotherapeutic for co-delivering an anti-inflammatory agent, piceatannol, and an anti-thrombotic agent, low molecular weight heparin (LMWH), to combat tumor metastasis by hindering the multiple steps of tumor metastasis. Piceatannol phosphate (PP) was synthesized through the phosphorylation of the hydroxyl groups of piceatannol. PP was then precipitated with calcium to form calcium phosphate nanoparticles (CaPP), which were further decorated with LMWH via electrostatic interaction on the surface to form a systemic metastasis-targeted nanotherapeutic (H@CaPP). LMWH possesses high affinity to P-selectin that is overexpressed on both activated endothelial cells (ECs) in pre-metastatic niche and activated platelets. Therefore, LMWH served as not only a targeting ligand to P-selectin but also an anti-micro-thrombi component. And PP successfully impeded the EMT process via downregulating vimentin and upregulating E-cadherin in invasion phase; hindered the pre-metastatic niche via downregulating intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, S100-A9 protein and metalloproteinase-9 in colonization phase (Fig. 1). When combined with chemotherapy or surgical resection, H@CaPP significantly reduced lung tumor metastasis and prolonged the overall survival of mice bearing triple-negative breast cancer. As proof of concept, this study combined anti-inflammatory drug and anticoagulant into a targeted nanotherapeutic, providing a safe and potential strategy for adjuvant therapy of metastatic tumors.
Minjun Xu, a PhD candidate of 2019 from School of Pharmacy, Fudan University, is the first author. Both Professor Jun Chen from School of Pharmacy, Fudan University and Professor Xiaoling Gao from School of Medicine, Shanghai Jiao Tong University, are the corresponding authors. This work was supported by National Natural Science Foundation of China.
Link address:https://www.nature.com/articles/s41467-021-23466-5
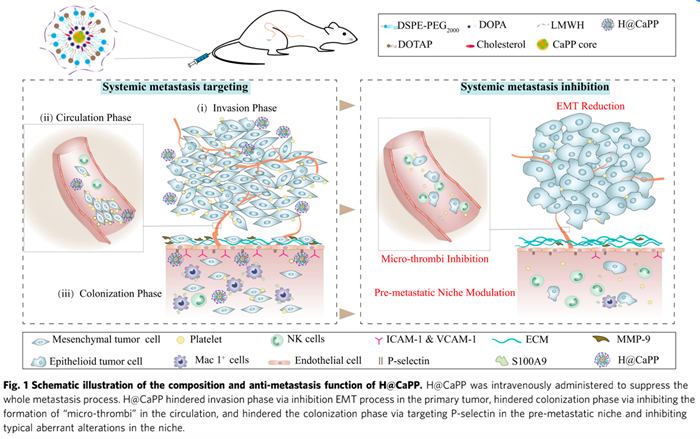
Figure 1. Schematic illustration of the composition and anti-metastasis function of H@CaPP