Recently, Professor Chen Jiang from School of Pharmacy, Fudan University, has developed a biomimetic dendrimer-peptide nanoconjugate, which had the function of regulating the pro-inflammatory microenvironment of brain lesions and was used for early multi-target treatment of Alzheimer’s disease (AD). The relevant studies were published online in Advanced Materials (IF=27.394), named entitled “Biomimetic Dendrimer-Peptide Conjugates for Early Multi-Target Therapy of Alzheimer’s Disease by Inflammatory Microenvironment Modulation”.
Current strategy for the treatment of AD is mainly focused on the “β-amyloid (Aβ)” hypothesis. Due to the chronic, long-term, and irreversible damage in the advanced stage of AD, it is difficult to achieve desired results in clinical trials which were conducted on this target. Therefore, reasonable and effective intervention for the early stage of AD progression is a promising treatment strategy.
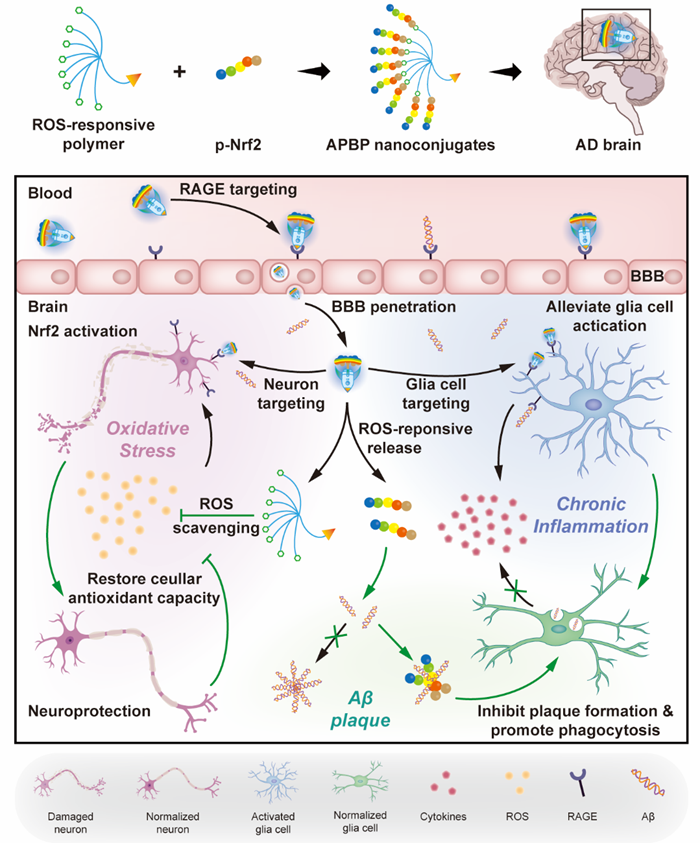
Figure 1. Illustration of APBP nanoconjugate formation and AD microenvironment modulation.
Due to the complexity of brain diseases, researchers have gradually realized that changes in the microenvironment of brain lesions have important guiding significance for the treatment of brain diseases. The early AD microenvironment is characterized by inflammatory responses and oxidative stress, which were reported to precede the appearance of the amyloid cascade. On the one hand, as the innate immune cells in the brain, microglia are of great significance for maintaining the metabolism and immune balance in the brain. Therefore, microglia can be treated as the core of modulation in the early stage of AD. On the other hand, continuously activated glial cells lead to the excessive production of reactive oxygen species (ROS) and further induce neuronal apoptosis. Thus, it is a feasible treatment to scavenge ROS accumulated in the microenvironment and restore the antioxidant capacity of nerve cells. Based on the characteristics of AD microenvironment, this study was designed and constructed a dendrimer-peptide nanoconjugate which could mimic the transport of Aβ in the brain to achieve multi-target modulation of microenvironment in the early stage of AD (Figure 1). The nanoconjugate could enter AD lesions efficiently, and scavenged ROS in the microenvironment through ROS-sensitive polymer materials. In addition, the conjugated peptide could activate nuclear factor E2-related factor 2 (Nrf2), thereby restoring the antioxidant capacity of damaged cells. Through the combined effects of the materials and peptide, this nanoconjugate could relieve oxidative stress fundamentally, reduce glial cell activation, polarize the transformation of M2 microglia, promote Aβ clearance, and finally realize the normalization of the microenvironment.The design ideas of this delivery system was verified in APP/PS1 transgenic mice and significant improvement of the cognitive function of AD model mice was achieved. This strategy provided a new perspective for AD treatment.
Peixin Liu, a PhD student of 2019 from School of Pharmacy, Fudan University, is the first author, while Professor Chen Jiang is the corresponding author. The work was supported by the grants from the international cooperative project of the National Key R&D Program of China (grant No. 2017YFE0126900), Program of Shanghai Academic Research Leader (18XD1400500), and Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZJLab. The authors also deeply thank Tamas Letoha from Pharmacoidea Ltd., Hungary, for the instruction during the experimental procedures.
Link address: https://onlinelibrary.wiley.com/doi/10.1002/adma.202100746