
Cancer metabolism plays multifaceted roles in the initiation and progression of tumors, and interventions in metabolism are considered fundamental approaches for cancer control. Within the vast metabolic networks of tumors, there exist numerous potential therapeutic targets, intricately interconnected with each other and with signaling networks related to immunity, metastasis, drug resistance, and more. Based on the characteristics of the tumor microenvironment, constructing drug delivery systems for multi-level modulation of the tumor microenvironment has been proven as an effective strategy for achieving multidimensional control of cancer. Consequently, we summarized several features of tumor metabolism to provide insights into recent advancements in intelligent drug delivery systems for achieving multi-level regulation of the metabolic microenvironment in cancer, with the aim of offering a novel paradigm for cancer treatment.The related results, titled Intelligent Delivery Systems in Tumor Metabolism Regulation: Exploring the Path Ahead, were published online in the Advanced Materials (IF=29.4).
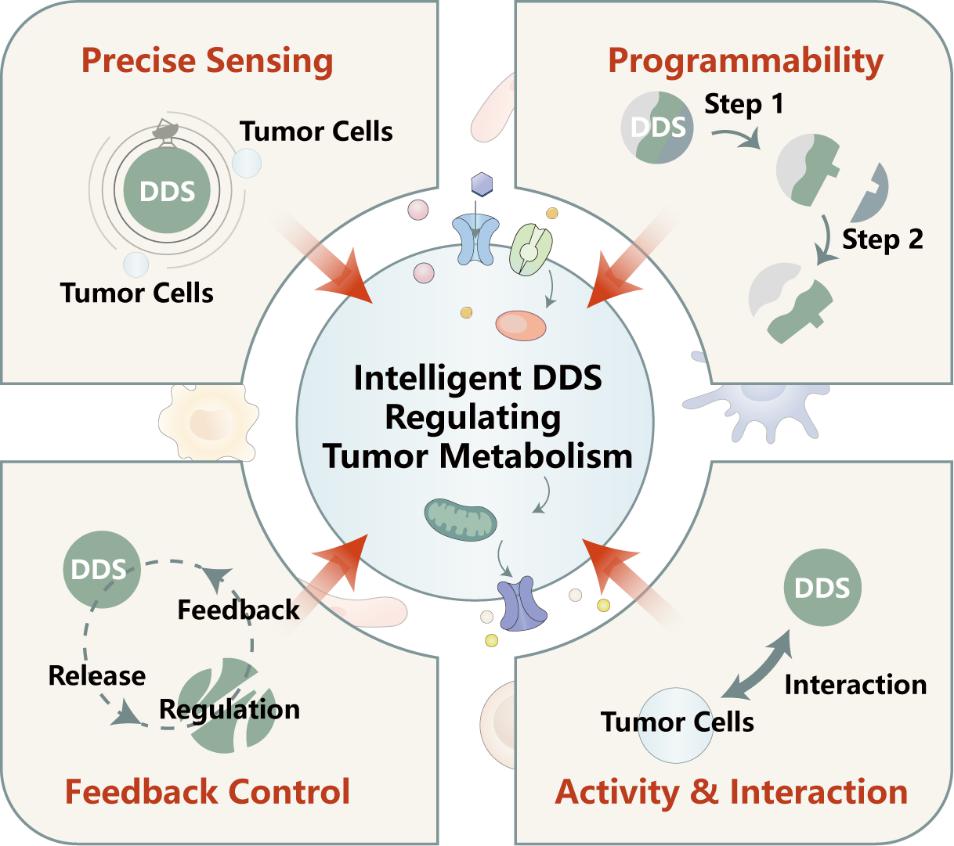
TOC: Latest advancements in intelligent drug delivery systems (DDS) exhibit distinctive advantages, including precise sensing, programmability, feedback control, and intrinsic activity. These attributes render them conducive to facilitating multi-dimensional therapeutic approaches grounded in metabolic regulation.
Malignant cells exhibit an adaptive metabolic reprogramming to fulfill their heightened bioenergetic and biosynthetic demands during their development [1].Metabolic processes involve the transformation and exchange of substances, driving intracellular and intercellular signaling communication, enabling tumor cells to adapt to various external pressures. These metabolic alterations encompass a wide array of aspects, including glucose metabolism, lactate metabolism, amino acid metabolism, and lipid metabolism. Accumulating evidence underscores the close association between aberrant tumor metabolism and drug resistance, immune suppression, and epigenetic mutations [2–4]. Hence, the need for therapeutic strategies based on tumor metabolic characteristics becomes increasingly pressing.
Metabolic processes of substances can be broadly categorized into three main phases: substrate uptake, enzyme-catalyzed reactions in metabolic pathways, and the efflux or utilization of metabolic products.Disturbing these three processes can impede the tumor's acquisition of nutrients or induce the accumulation of toxic byproducts, disrupting the intracellular and extracellular equilibrium. Although several small molecule drugs have been developed for these purposes and are in clinical trials (2-deoxy-D-glucose, NLG919, etc.), they face certain limitations. Firstly, the metabolic processes of tumor cells are often interconnected across multiple pathways, implying that the regulation of a single pathway may be influenced by others. Furthermore, some metabolic pathways are shared between normal and tumor cells, leading to unexpected side effects when drugs accumulate in normal tissues. Hence, there is an urgent need to explore more precise and efficient therapeutic strategies to address the intricacies of tumor metabolism.
The effectiveness of metabolic regulation strategies is expected to be enhanced through the rational design of delivery systems. Intelligent delivery systems possess advantages such as precise sensing, programmability, and feedback control, making them conducive to supporting multi-dimensional therapeutic approaches based on metabolism [5–7]. To gain a comprehensive understanding of tumor metabolism from a therapeutic perspective and provide guidance for the design of delivery systems, we have focused on examining transport and conversion pathways that can be harnessed for intervention. We have summarized the general characteristics of tumor metabolism, categorized known metabolic vulnerabilities and cross pathways, and outlined the fundamental concepts for intelligent delivery system design for metabolism regulation. It is our hope that these efforts will inspire the development of various combinatorial therapeutic strategies.

Scheme 1. Intelligent delivery systems in tumor metabolism regulation.
Xuwen Li, a doctoral student (member of the Zhuo Bo Program) in our research group, is the first author of the paper, while Associate Professor Tao Sun and Professor Chen Jiang are the corresponding authors. This study has received support from projects such as the National Natural Science Foundation of China and the Shanghai Academic Research Leaders Program and other projects.
Original link: https://onlinelibrary.wiley.com/doi/10.1002/adma.202309582