
In recent years, the immune regulatory function of retinoic acid receptor related orphan receptor RORγt in tumor microenvironment (TME) has attracted considerable attentions, and RORγt has become a highly promising drug target for cancer immunotherapy. Some studies have demonstrated that T helper 17 (Th17) cells and the secreted cytokine interleukin-17 (IL-17) widely exist in various TMEs, which inhibit tumor proliferation by promoting significant activation of tumor-specific CD8+ cytotoxic T cells (Tc17 cells). RORγt is specifically expressed in Th17 and Tc17 cells and is a key transcription factor that regulates the differentiation of CD4+ T cells into Th17 cells, the production of Tc17 cells and the secretion of IL-17A. RORγt agonists can promote Th17 cell differentiation, IL-17A production and Tc17 cell activation, thereby exerting anti-tumor immune effects. Therefore, development of novel RORγt agonists is of great significance for small molecule cancer immunotherapy.
Prof. Yonghui Wang's research group in School of Pharmacy has been making continuous efforts to discover novel RORγt modulators and understand the mechanisms of action (MOAs). They have reported various RORγt modulators of different structural types, including RORγt inhibitors (or inverse agonists) for the treatment of autoimmune diseases and RORγt agonists for cancer immunotherapy. Moreover, they have revealed molecular mechanisms underlying the inhibition-activation functional switch of RORγt, and published two review articles in the Journal of Medicinal Chemistryin 2018 and 2021, respectively. (Miniperspective, J. Med. Chem. 2018, 61, 5794−5804; Perspective, J. Med. Chem.2021, 64, 10519–10536.)
Recently, the research group of Assoc. Prof. Qiong Xie and Prof. Yonghui Wang published a new research article entitled "Discovery of Biaryl Amide Derivatives as Potent, Selective, and Orally Bioavailable RORγt Agonists for Cancer Immunotherapy " in Journal of Medicinal Chemistry. Therein, a series of biaryl amide derivatives was discovered as novel small molecule RORγt agonists, which not only displayed potent and selective in vitro agonistic activity on RORγt, but also demonstrated excellent in vivo pharmacokinetic (PK) profiles and good single-drug in vivo anti-tumor efficacy in both mouse melanoma and lung adenocarcinoma tumor models, which indicated future development potentials and clinical application prospects.

Figure 1. Discovery and optimization of novel biaryl amide RORγt agonists
Starting from RORγt inverse agonist GSK805 (1), a “functionality switching” strategy was employed by introducing para-substituents of varying sizes to the left-hand side (LHS) phenyl ring, resulting in the discovery of an isobutyl-substituted RORγt agonist 2. Compound 2 has good in vitro RORγt agonistic activity (EC50 = 12.2 nM), but poor liver microsomal stability (HLM: t1/2 =2.6 min) and low oral bioavailability (mice, F = 4.1%). In order to optimize the PK properties while maintaining agonistic potency, the LHS, linker, and the right-hand side (RHS) fragments of the biaryl amide compounds were fine-tuned using strategies like bioisosterism, which led to the discovery of a promising RORγt agonist candidate compound 14(Figure 1), superior to the Phase II clinical compound LYC-55716. The isopropyloxy-substituted compound 14 has strong RORγt agonistic potency (RORγt dual FRET: EC50 = 10.8 nM; mTh17: EC50 = 8.2 nM), high RORγ subtype selectivity (> 100 fold), good liver microsomal metabolic stability in different species (mouse, dog, rat, and human liver microsomes: t1/2>145 min) and excellent oral bioavailability (F = 90.3% (mouse), 39.4% (rat), 52.2% (dog)).
In vivo pharmacodynamic (PD) evaluation and MOA studies in collaboration with Prof. Di Zhu’s group from School of Basic Medical Sciences revealed that compound 14 showed robust in vivo anti-tumor activity (Figure 2). In preclinical tumor models of mouse B16F10 melanoma and mouse LLC lung adenocarcinoma, compound 14 showed tumor growth inhibition (TGI) rates of 77.9% (100 mg/kg, i.p., Figure 2A) and 75.6% (50 mg/kg, i.p., Figure 2B), respectively. Significant increases of the proportion of IL-17A+ cells in CD3+ T cells and CD4+ T cells (Figure 2C-D), as well as the expressions of Rorc and Il-17a genes (Figure 2E-F), were observed in compound 14-treated (25 mg/kg, i.p.) LLC tumors. Compound 14 had low risk of hERG-mediated cardiac toxicity and showed no mutagenic effect in the mini-AMES test, indicating that compound 14 deserves further investigation as a potential drug candidate of RORγt agonist for cancer immunotherapy.
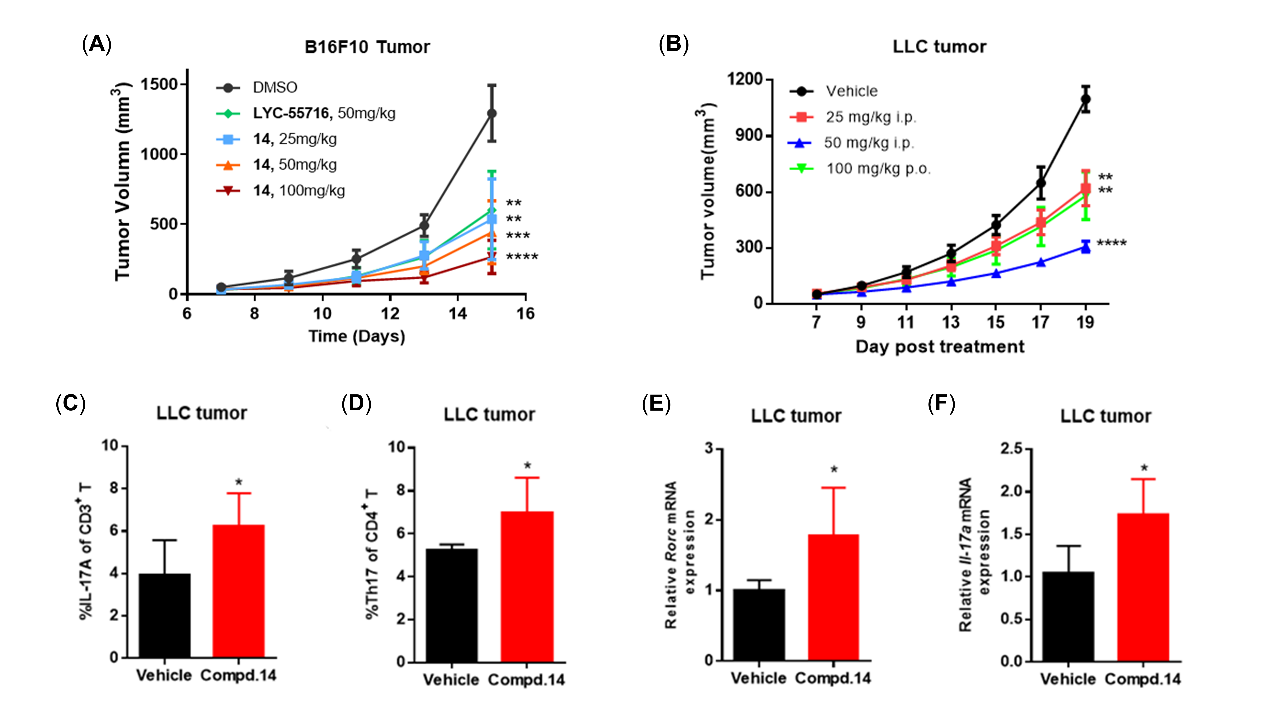
Figure 2. In vivo efficacy of candidate compound 14 in mouse B16F10 (A) and LLC (B)tumor models, and changes in the IL-17A+ cell ratios in CD3+ T cells (C) and CD4+ T cells (D) and in the expression levels of Rorc (E) and Il-17a (F) genes in compound 14-treated LLC tumors.
Molecular docking study showed that the sulfone group of compound 14 is associated with key hydrogen bonds with Leu287 and Arg367 in RORγt ligand binding domain (LBD), and the NH in amide linker forms a hydrogen bond with the carbonyl group of Phe377. Three benzene rings in compound 14 form π-π stacking interactions with Phe377, Phe378, and Phe388, respectively (Figure 3A). To understand the role of the isopropoxy group of agonist compound 14 in stabilizing the active state of activation function 2 (AF2) domain and switching the function of RORγt from inverse agonism into agonism, a molecular dynamics (MD) simulation study was carried out in collaboration with Assoc. Prof. Yan Li’s group from School of Pharmacy. Due to the presence of an unoccupied cavity around the para-position of the LHS phenyl ring of inverse agonist 2b, Tyr502‘s side chain shifted outward, which led to the disruption of the His479-Tyr502 hydrogen bond (Figure 3C). On the other hand, compound 14 can stabilize the hydrogen bond between His479 and Tyr502 (Figure 3B), which is crucial for stabilizing the agonistic conformation of AF2 helix 12 (H12) and maintaining the agonism function of RORγt.
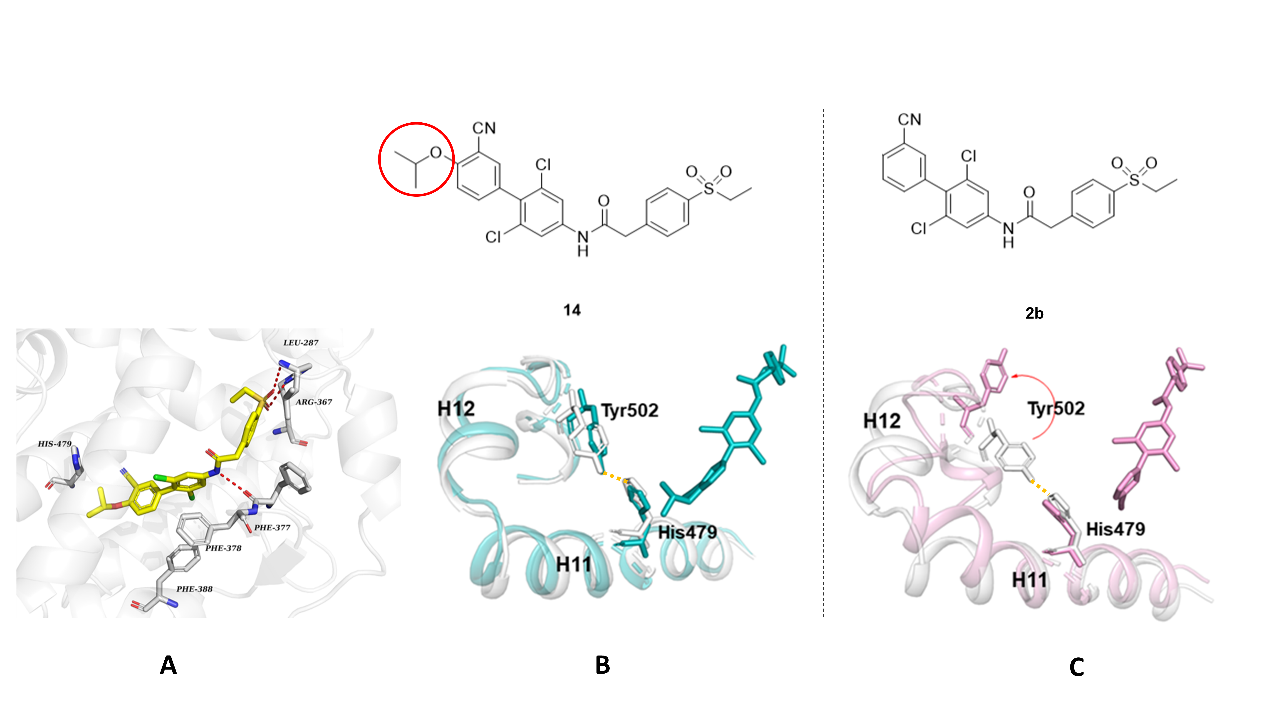
Figure 3. Docking mode of compound 14 in RORγt LBD (A), and MD simulation results of agonist 14 (B), and inverse agonist 2b (C) in RORγt LBD (the initial conformation of the protein H11-H12 is shown in white, and the conformation snapshot at 1000 ns is shown in blue-green and pink, respectively.)
Lixue Lu (a Ph. D candidate), Dr. Yafei Huang and the research assistant Meiqi Song from the Department of Medicinal Chemistry, School of Pharmacy shared the co-first authorship of this article. Assoc. Prof. Qiong Xie and Prof. Yonghui Wang are the co-corresponding authors. The work was funded by the National Natural Science Foundation of China, the National Science and Technology Major Project, and the Shanghai Biopharmaceutical and Technology Supporting Plan from Shanghai Municipal Science and Technology Commission. Novel compounds have been granted patents in China (CN108863850B), the United States (US10844017B), and Japan (JP6953538B2).
The full article is available through the link:https://pubs.acs.org/doi/epdf/10.1021/acs.jmedchem.3c01492
Introduction of the Research Group of Prof. Yonghui Wang and Assoc. Prof. Qiong Xie
The research group of Prof. Yonghui Wang and Assoc. Prof. Qiong Xie from the School of Pharmacy, Fudan University mainly focuses on the discovery of small molecule drugs for autoimmune diseases and tumor immunotherapy, and the research on new methods and technologies of organic and medicinal chemistry. Wang’s group is currently composed of 1 chair professor, 1 associate professor, 3 Ph. D. students and 4 master students. In recent years, 7 graduates have obtained their Ph. D. degrees and 5 graduates have obtained their master’s degrees in this group. The research group has been funded more than 20 scientific research projects by the National Science and Technology Major Project, the National Natural Science Foundation of China, the Shanghai Biopharmaceutical and Technology Supporting Plan from Shanghai Municipal Science and Technology Commission, and the Natural Science Foundation of Shanghai, with a cumulative funding of more than 11 million yuan. In the past 5 years, Wang’s group has published more than 25 SCI papers in academic journals such as J Med Chem, Eur J Med Chem, J Org Chem, ACS Med Chem Lett, and applied for 16 Chinese patents (6 authorized) and 2 international PCT patent applications in the United States, Japan, and Europe (6 international patents have been authorized). In the research of small molecule drugs to treat autoimmune diseases and cancers, a number of drug leads and candidates with potentials for further development have been discovered and are undergoing preclinical evaluation.
