
Recently, Yonghui Wang’s group (School of Pharmacy, Fudan University) and Weiqiang Lu’s group (School of Life Science, ECNU) published an article entitled ‘Design, Synthesis, and Bioevaluation of 2-Aminopteridin-7(8H)-one Derivatives as Novel Potent Adenosine A2A Receptor Antagonists for Cancer Immunotherapy’ in Journal of Medicinal Chemistry. In this article, a new class of small molecule A2AR antagonists with the core structure of pteridone was discovered, which showed therapeutic potential for cancer immunotherapy.
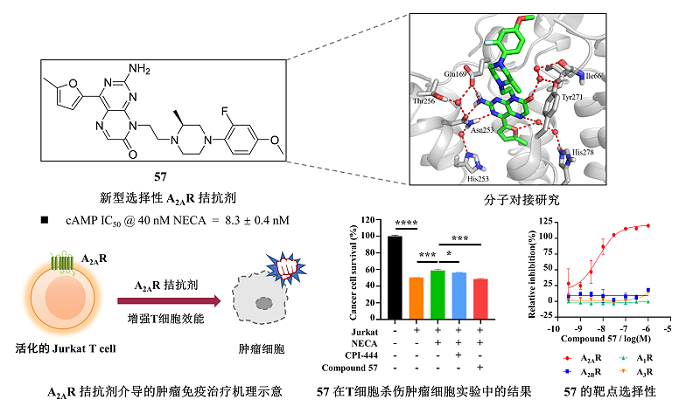
Chemical structure, docking model, activity and selectivity of the pteridone-based A2AR antagonist compound 57 and its effect on enhancing T cell activation and tumor cell killing activity.
In recent years, the regulatory function of adenosine A2A receptor (A2AR) in tumor immunology has attracted extensive attention, and A2AR has become a promising emerging target for cancer immunotherapy. The A2AR signaling pathway inhibits T cell activation and effector function, which impairs antitumor immune responses and induces tumor immune escape. Recently, a number of A2AR small molecule antagonists have been investigated in relevant clinical anti-tumor studies. However, the concentration of adenosine in the tumor microenvironment (TME) is usually at the micromolar level, which is 10-100 times higher than the concentration in the normal physiological environment, and also exceeds the concentration in the pathological state of Parkinson's disease (PD), which greatly limits the efficacy of A2AR antagonists in tumor immunotherapy. Therefore, the development of novel A2AR antagonists that can maintain high activity in a high concentration of adenosine is of great significance for cancer immunotherapy.
In this study, a hit compound with A2AR antagonistic activity at μM level was identified through in-group compound screening. Drug design strategies such as scaffold hopping and conformational restriction were used for multiple rounds of optimization on the hit. A lead compound 57 with nM level of A2AR antagonistic activity was discovered (IC50 = 8.3 nM), which is 10-fold more active than the clinical phase II compound CPI-444 (IC50 = 86.5 nM). Compound 57 is a highly selective antagonist of A2AR, with over 1000-fold selectivity for A1R, A2BR, and A3R. Further studies found that 57 maintained the activity (IC50 = 179.6 nM) at a TME-mimicking condition with 1 μM NECA (5'-N-ethylformamidoadenosine, a potent A2AR agonist).
Further studies indicated that compound 57 could significantly enhance T cell activation and tumor cell-killing activity. The cytokine IL-2 is an important biomarker of T cell activation. When the A2AR on T cells was activated by NECA, the IL-2 mRNA and protein expression levels were significantly decreased. Adding compound 57 to antagonize A2AR, the expression level of IL-2 can be significantly restored. Similarly, in the co-culture experiment of tumor cells and T cells, 57 significantly reversed the inhibitory effect of NECA on the T cells’ tumor killing ability. In conclusion, compound 57 shows a certain potential on tumor immunotherapy, which provides a new starting point for the development of anti-cancer drugs targeting A2AR.
Fazhi Yu (Ph.D., School of Pharmacy, Fudan University), Chenyu Zhu (a master candidate from the School of Pharmacy, Fudan University) and Shuyin Ze (ECNU) shared the co-first authorship. Prof. Yonghui Wang and Assoc. Prof. Qiong Xie (School of Pharmacy, Fudan University) and Assoc. Prof. Weiqiang Lu (School of Life Science, ECNU) are the co-corresponding authors. This work was funded by the National Natural Science Foundation of China, the Natural Science Foundation of Shanghai, and the National Science and Technology Major Project.
Original link: https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c02199
Introduction of Yonghui Wang’s Group
The research group of Prof. Yonghui Wang (School of Pharmacy, Fudan University) mainly focuses on the discovery of small molecule drugs for autoimmune diseases and tumor immunotherapy, and the research on new methods and technologies of organic and medicinal chemistry. Wang’s group is currently composed of 1 professor, 2 associate professors, 4 Ph. D. students and 4 master students. In recent years,5 students obtained their Ph. D. degrees and 4 students obtained their master’s degrees in this group. The research group has been funded more than 20 scientific research projects by the National Science and Technology Major Project, the National Natural Science Foundation of China, Shanghai Biopharmaceutical and Technology Supporting Plan, and the Natural Science Foundation of Shanghai, with a cumulative funding of more than 11 million yuan. In the past 5 years, Wang’s group has published more than 20 SCI papers in academic journals such as J Med Chem, Eur J Med Chem, J Org Chem, ACS Med Chem Lett, and applied for 13 Chinese patents (5 authorized) and 2 international PCT patent applications in the United States, Japan, and Europe (5 international patents have been granted). In the research of small molecule drugs to treat autoimmune diseases and cancers, a number of drug candidates with potentials for further development have been discovered and are undergoing preclinical evaluation.
