
Cardiovascular disease (CVD) is a leading cause of death and loss of productive life years worldwide1. Atherosclerosis (AS) is the underlying pathological cause for the development of CVDs and the main contributor to cardiovascular mortality2. The predominant cells present in atherosclerotic plaques are inflammatory macrophages3. Circulating monocytes derived macrophages rely on scavenger receptors (mainly CD36) to uptake modified lipoproteins and turn into lipid-laden cells3. These lipid-laden macrophages---foam cells, lead to plaque formation and secrete inflammatory mediators that persist inflammation and promote plaque progression4. Within plaques, macrophages show a profound metabolic reprogramming which is driven by atherogenic factors in the plaque microenvironment, such as cytokines, oxidized lipids, and hypoxia5. Growing evidence suggested that metabolic reprogramming of macrophages is a therapeutic approach to treat AS6,7. However, the regulatory mechanisms controlling macrophage repolarization has not been fully illuminated.
In July 31st, 2020, the top journal in the field of cardiology published the study “Sorting Nexin 10 Mediates Metabolic Reprogramming of Macrophages in Atherosclerosis through the Lyn-dependent TFEB Signaling Pathway, Circulation Research, 2020, 127:534-549”. This work was co-led by Professor Xiaoyan Shen at School of Pharmacy, Fudan University, Researcher Mingyue Zheng at Shanghai Institute of Materia Medica and Professor Lefeng Qu at Shanghai Changzheng Hospital.
This new finding shed light on SNX10 as a negative regulator of FAO and OXPHOS in macrophages. Its deficiency promoted an anti-inflammatory phenotype and limited foam cell formation. In mice, myeloid cell-specific ablation of SNX10 alleviated AS characterized decreased inflammation, foam cell formation and necrotic core formation in the lesions. Mechanistically, decreased foam cell formation by the absence of SNX10 was linked to the inhibition of CD36 internalization. Through interacting with Lyn-AKT, SNX10 mediates CD36 internalization, while SNX10 deficiency interrupts Lyn-dependent AKT activation, leading to increased TFEB-mediated lysosomal biogenesis and mitochondrial oxidative phosphorylation, and thereby promotes IRF4 induced anti-inflammatory type macrophage polarization to inhibit atherogenesis and inflammatory response.
This work has identified SNX10 might be a potential target for AS therapy that activate a key braking mechanism against the inflammatory response and lipid accumulation. It marks a critical step toward developing a potential new therapy that induces an anti-inflammatory metabolic state in macrophages that protects against AS.
Yan You, postdoctoral fellow in NIH and Weilian Bao, member of Professor Shen’s lab are the first authors. Professor Xiaoyan Shen at School of Pharmacy, Fudan University, Researcher Mingyue Zheng at Shanghai Institute of Materia Medica, and Professor Lefeng Qu at Shanghai Changzheng Hospital are the corresponding authors.
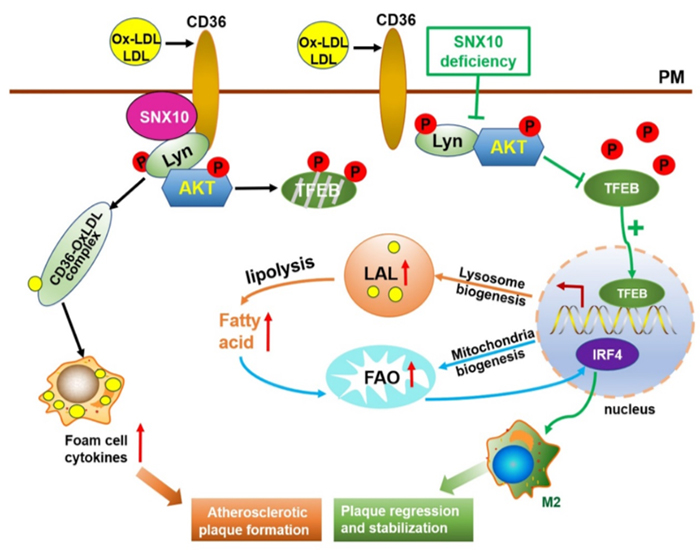
Figure 1. Sorting nexin 10 mediates metabolic reprogramming of macrophages in AS through the Lyn-dependent TFEB signaling pathway
References
1.Sergin I, Zhang XY, Evans TD, et al. Exploiting Macrophage AutophagyLysosomal Biogenesis With the Natural Sugar Trehalose as a Therapy for Atherosclerosis[J]. Arteriosclerosis Thrombosis and Vascular Biology, 2017, 37.
2.Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R and Moran AE. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch Med Res. 2015;46:328-38.
3.Witztum JL and Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9: 73-102.
4.Tabas I, Lichtman A H. Monocyte-Macrophages and T Cells in Atherosclerosis[J]. Immunity, 2017, 47(4): 621-634.
5.Tabas I and Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res. 2016;118: 653-67.
6.Koelwyn GJ, Corr EM, Erbay E and Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526-537.
7.Ouimet M, Ediriweera HN, Gundra UM, et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125:4334-48.