
Recently, Professor Wei Lu from School of Pharmacy, Fudan University, has designed a new theranostic preparation using “hitchhiking technique” for the intraoperative fluorescence image-guided cancer photodynamic and checkpoint blockade therapy. The work has been published entitled “High affinity of Chlorin e6 to immunoglobulin G for intraoperative fluorescence image-guided cancer photodynamic and checkpoint blockade therapy” in ACS Nano.
As the emerging therapy, immunotherapy, especially the checkpoint blockade therapy, has achieved significant clinical outcome, which holds promise for the cure of cancer. However, the overall response rate of the patients treated with checkpoint blockade monotherapy was only 20-30%.
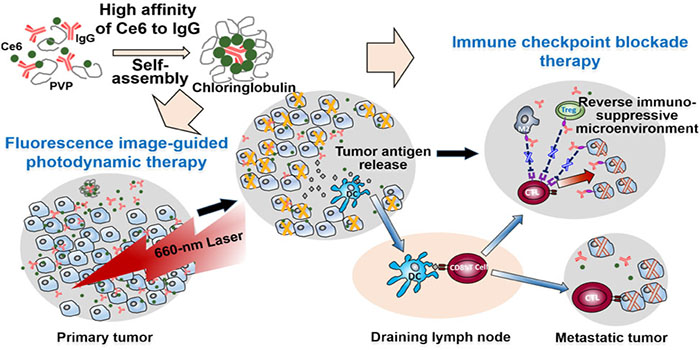
Photodynamic therapy (PDT) is a medical treatment which utilizes none or low toxic photosensitizers and light source to produce reactive oxygen species to kill the tumor cells. It is a highly specific and safe local therapeutic modality. For small tumor residuals that are not completely removed by surgery, the postsurgical PDT can be used successfully in reducing the risk of recurrence and metastasis. More importantly, PDT triggers cancer cells to release tumor antigens and induces the host anti-tumor immune response. Recent animal studies have shown that PDT combined with the checkpoint blockade therapy elicit synergistic effects for the treatment of cancer.
In order to increase the efficacy of the photodynamic therapy, the photosensitizer is generally designed to be entrapped into the nanostructures which increase the tumor accumulation through their prolonged blood circulation. However, such design slows down the clearance of the photosensitizer from the body. Therefore, patients have to avoid direct sunlight or bright indoor light for a very long time of period in order to prevent the phototoxicity.
The research group occasionally found that immunoglobulin G (usually called antibody) inherently bound to the photosensitizer Chlorin e6 in a high affinity. They developed a so-called “hitchhiking technique” to use the antibody to carry the photosensitizer to the tumor without changing its clearance rate in blood. This preparation method allows to co-deliver single or multiple checkpoint inhibitor antibodies and the photosensitizer in a single setting for the intraoperative fluorescence image-guided cancer photodynamic and the checkpoint blockade therapy.
The research groups prepared a theranostic formulation that combined the photosensitizer and anti-PD-L1 antibody for the treatment of mice bearing glioma and demonstrated a combinatorial fluorescence image-guided surgery, which offered more accurate tumor resection, and PDT and PD-L1 blockade, together significantly prolonging the survival of the mice. In addition, in mice bearing colon cancer, the researchers used anti-PD-L1 antibody and anti-CTLA-4 antibody, to formulate the preparation. The formulation allowed the intraoperative fluorescence image-guided PDT of both primary and metastatic lesions with size as small as 1 mm in combination with PD-L1 and CTLA-4 dual checkpoint blockade, which significantly extended the life-span of mice and elicited a long-lasting immune memory effect against tumor recurrence. The technology developed paves a new way for combinatorial intraoperative fluorescence image-guided surgery, PDT and checkpoint blockade therapy and shows great potential for clinical translation.
Web link: https://pubs.acs.org/doi/10.1021/acsnano.9b03466