
With the support of National Science Fund for Distinguished Young Scholars and National Natural Science Foundation of China, Prof. Chen Jiang’s group from Fudan University, jointed with Prof. Weigen Lu in China State Institute of Pharmaceutical Industry, led PhD student Yifei Lu and others who developed a polymeric micelle drug delivery system with novel functions in neurovascular unit remodeling and treating acute ischemic stroke (Figure 1). The study was published on the high impact journal Advanced Materials entitled ‘Microthrombus-Targeting Micelles for Neurovascular Remodeling and Enhanced Microcirculatory Perfusion in Acute Ischemic Stroke’, and aroused attention in the field.
Stroke can be categroized into hemorrhagic and ischemic stroke, with acute ischemic stroke being the major type in clinic (up to 70%). Due to the well-established thrombolytic therapy, mortality of stroke has significantly decreased, but still nearly 40% of survivors are disabled after stroke onset despite of complete recanalization. This is mainly attributed to the reperfusion injury followed with the restoration of blood flow, causing further deterioration of brain infarction and limited blood perfusion called ‘no-reflow’ phenomenon. Thus, effective strategy against reperfusioin injury of ischemic stroke is on demand.
As said by Prof. Chen Jiang, a new research model named neurovascular unit (NVU) has been proposed as a basic structure for understanding brain pathologies. NVU includes neurons, endothelium cells, microglia and other various brain cell types. During ischemia and reperfusion, complex cellular cascades including oxidative stress, neuroinflammation and brain vascular impairment occur, leading to abnormal metabolism, phenotype shift and function changes of multiple cell types in NVU, which cause the irreversible brain damage.
To solve the problems, the research group developed a micelle drug delivery system targeting to the microthrombus in the injured brain vasculature and encapsulated rapamycin to modulate the cellular stress pathway in order to achieve simultaneous modulation of multiple cell types in NVU (Figure 2). The micelles could bind to microthrombus and penetrate the impaired blood-brain barrier (BBB) into the ischemic lesion. Drug release could be triggered by the high level of reactive oxygen species (ROS) in the oxidative microenvironment, meanwhile effectively eliminate ROS; the released rapamycin could enhance the anti-stress autophagy and polarize microglia to the M2 repairing phenotype. By combining the functions of carriers and drugs, the polymeric micelles could achieve effective neuroprotection, microglia polarization and restoration of BBB function, thus remodeling the neurovascular unit to improve the microcirculatory perfusion and reduce brain infarction.
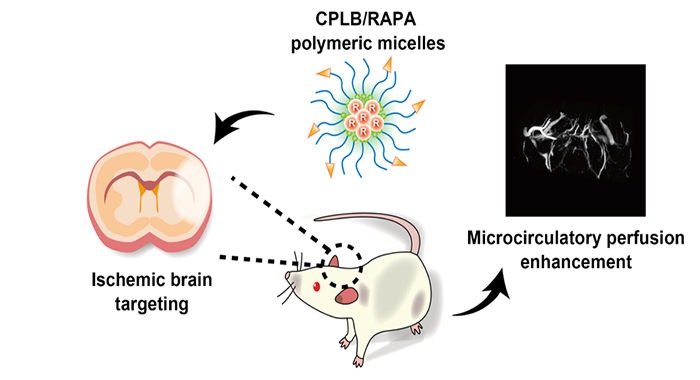
Figure 1. Novel polymeric micelles targeting to ischemic brain, alleviating reperfusion injury and enhancing microcirculatory perfusion
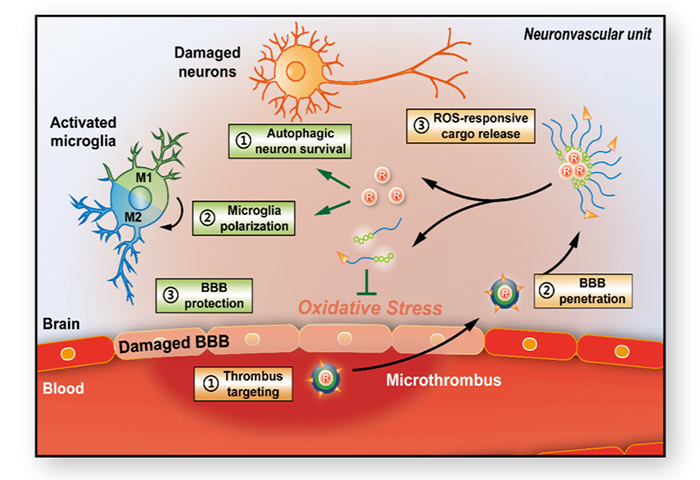
Figure 2. Mechanism of the multi-cell type modulation by polymeric micelles CPLB/RAPA