
The limited chemotherapy effect of pancreatic cancer is related to its unique and complex tumor microenvironment (TME). The presence of dense extracellular matrix leads to the low permeability of traditional chemotherapy drugs, and the high toxicity of chemotherapy drugs restricts their clinical application. Therefore, it is necessary to explore new methods to improve the therapeutic effect of pancreatic cancer by regulating TME and selecting more effective and safer therapeutic drugs.
The TME of pancreatic cancer contains various mesenchymal cells and dense extracellular matrix, which together form a solid barrier to prevent drug delivery and penetration into the tumor. The formation of this barrier is associated with the activation of cancer-related fibroblasts (CAFs) by transforming growth factor (TGF-β) signaling in TME. On the other hand, KRAS mutation in pancreatic cancer cells is the marker of the initiation and progression of 90% pancreatic cancer. Due to the undruggable nature of KRAS mutation, chemotherapy drugs used currently in clinic could not interfere with it. Besides, the relatively high systemic toxicity of chemotherapy drugs resulted in poor prognosis hence hindered its application in treatment. Professor Jun Chen’s group from School of Pharmacy of Fudan University and Professor Xiaoling Gao’s team from Shanghai Jiao Tong University collaborated to report a study of the sequentially combination of TME regulation and cancer cell target intervention in the treatment of pancreatic cancer. Firstly, the natural product fraxinellone with TGF-β regulatory effect was screened and encapsulated in targeted nanomaterials. The formed nanoparticles effectively inhibited the activation of CAFs, reduced the secretion of collagen, promoted the normalization of tumor blood vessels and facilitated the infiltration of the subsequent nanoparticles in tumor sites. Secondly, the biomimetic high-density lipoprotein nanoparticles were designed and constructed in order to silence KRAS mutation. The nanoparticles were absorbed by pancreatic cancer cells in large quantities and then successfully escaped from lysosomes into the cytoplasm to play the role of gene silencing. In the in situ model of pancreatic cancer, sequential administration of the two agents achieved high efficacy and low toxicity in anti-cancer treatment, and significantly prolonged the survival period of tumor-bearing nude mice. This study provides a promising method for the treatment of tumors with rich stroma.
Relevant studies were published online in Small entitled "Sequential Targeting TGF-β Signaling and KRAS Mutation Increases Therapeutic Efficacy in Pancreatic Cancer". Yuanyuan Pei, a master student from School of Pharmacy of Fudan University, is the first author of this paper. Prof. Jun Chen from School of Pharmacy of Fudan University and Prof. Xiaoling Gao from Shanghai Jiao Tong University are the co-corresponding authors. This work was supported by National Natural Science Foundation of China and “Shu Guang” project funded by Shanghai Municipal Education Commission and Shanghai Education Development Foundation.
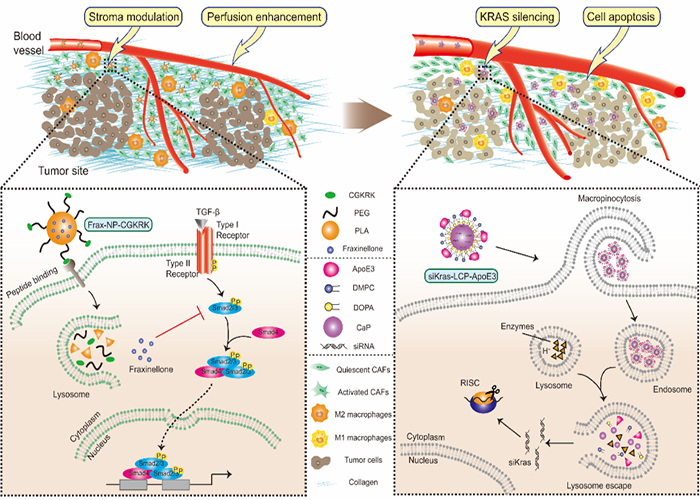
Illustration of sequentially targeting TGF-β signaling and KRAS mutation for pancreatic cancer therapy.