
Recently, Professor Wei Lu of the School of Pharmacy, Fudan University and Professor Zeyu Xiao of the School of Medicine, Shanghai Jiaotong University reported a new theranostic preparation of albumin-bound near-infrared II small molecule. By means of intraoperative near-infrared fluorescence imaging, the preparation enables to identify primary tumors and micrometastases, and photothermal therapy under the guidance of images to thoroughly remove tiny tumors, thus avoiding the recurrence of postoperative tumors. The research results were published online on May 17th in Nature Communications with the title "Albumin tailoring fluorescence and photothermal conversion effect of near-infrared-II fluorophore with aggregation-induced emission characteristics" (https://www.nature.com/articles/s41467-019-10056-9/).
Tumors are diseases of high morbidity and mortality worldwide. Currently, surgical removal of tumors remains the most effective treatment. However, in many invasive or metastatic cancers, the tumor boundaries are not clear, and tumor cells infiltrate into vital organs and tissues. In these cases, even an experienced surgeon cannot completely remove the tumor by surgery, leaving residual tiny tumors. These tumors can cause lethal recurrence and metastasis. Therefore, to achieve intraoperative detection and removal of these residual microlesions is essential for cancer prognosis.
Intraoperative fluorescence imaging has the advantages of high resolution and fast imaging, which can guide surgeons to more clearly distinguish between small tumors and normal tissues during surgery. However, the excitation and emission wavelengths of intraoperative fluorescent probes currently in clinical use are in the visible range. The imaging depth is limited, and the tissue autofluorescence interference is considerable. If a near-infrared fluorescent probe molecule with deeper imaging depth and low background interference can be found, and if it also has the photothermal conversion performance of absorbing light energy into thermal energy and generating thermal ablation, the tiny tumor during the intraoperative detection can be treated with photothermal therapy. Therefore, the development of the preparations with both near-infrared fluorescent and photothermal conversion abilities is critical for the clinical diagnosis and treatment of microtumor.
The joint team thought of a small-molecule fluorescent probe capable of imaging at the near-infrared II region wavelength, 1000 nm to 1400 nm. When the probe is dissolved in a polar organic solvent, the fluorescence is quenched but exhibits high photothermal conversion performance. When the probe is aggregated in an aqueous solution, the photothermal conversion performance is decreased, but the fluorescence imaging performance is enhanced. Interestingly, the team also found that human serum albumin can bind to this small molecule probe with high affinity, thus restricting its molecular rotation and tailoring its fluorescence and photothermal properties. In addition, albumin as a carrier can specifically deliver and enrich the small molecule probe in the tumor area. Using mouse-bearing colon cancer with microtumor as a model, the preparation can achieve intraoperative fluorescence imaging of 0.5 mm × 0.3 mm micrometastases without interference from intestinal contents. Meanwhile, the spatial and temporal distribution of the preparation can be monitored by the fluorescence imaging to optimize the position, area, dose and time of the laser treatment, thereby achieving accurate photothermal removal of the microtumor during the operation. This study provides a new research approach for the clinically intraoperative real-time detection and photothermal removal of microtumor.
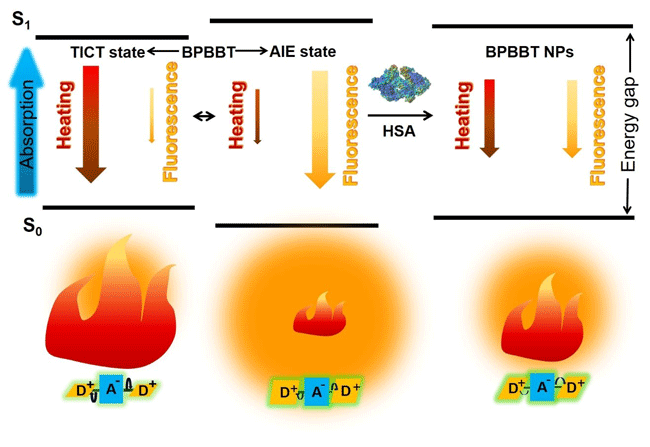
Diagram. HSA altering fluorescence and photothermal efficiency of BPBBT