
Epigenetic alteration like DNA hypermethylation plays a pivotal role in immune evasion of cancer cells during tumorigenesis. It is a common feature of heterogeneous cancer phenotypes for the reason that the tumor-associated antigen (TAA) promoter regions are hypermethylated in cancers, which lead to the low expression of TAAs.
The question is how to let the tricky tumor cells show their true colors? The researchers used Zebularine (Zeb), one of hypomethylating agents (HMAs), to up-regulate the expression of TAAs, increase tumor immunogenicity and enhance infiltration of CD8+ T cells. Additionally, Zebularine could regulate immunosuppressive tumor microenvironment (TME) by reducing myeloid-derived suppressor cells (MDSCs).
As is well-known, tumor cells are wolves in “PD-L1 clothing”, which could interact with the PD-1 receptor on activated T cells, resulting in T cell anergy. Therefore, combination of Zeb and PD-1 antibod is potential to enhance the antitumor immune response.
Recently, Prof. Weiyue Lu from Fudan University and Prof. Zhen Gu from UCLA worked together to engineer an in situ formed dual-bioresponsive depot to co-deliver these two agents. PD-1 antibody was first loaded in the pH-sensitive CaCO3 nanoparticles (aPD1-NPs) for locally sustained release, and then the aPD1-NPs and Zeb were encapsulated together into the ROS-responsive hydrogel (Zeb-aPD1-NPs-Gel). This dual-responsive scaffold enabled to achieve controlled release of payloads by responding to the acidic pH and ROS condition associated with TME, and then effectively boosted the T cell-mediated antitumor immune response.
Researchers validated that this combination strategy could enhance the inhibition of tumor growth and prolonged the median survival time of model mice bearing subcutaneous melanoma. Furthermore, local delivery of Zeb-aPD1-NPs-Gel could effectively induce the systemic antitumor immune. The mice model bearing melanoma tumor cells on both sides were constructed. A Zeb-aPD1-NPs-Gel was injected just next to the left tumor. The tumor growth on both sides had a similar tendency and was obviously inhibited. This delivery strategy integrated with both epigenetic modulators and immune checkpoint blockade treatments may be translated for enhancing objective response rates in clinic.
This work was recently published in Advanced Materials as a front cover. (DOI: 10.1002/adma.201806957).
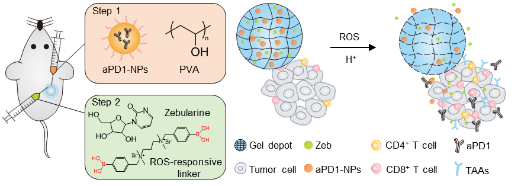
Schematicof an injectable in situ formed ROS/H+ dual bioresponsive gel depot