
Triple-negative breast cancer (TNBC) is an extremely hard-to-treat subtype (~15%) of breast cancer. As TNBC lacks expression of human epidermal growth factor 2 (HER2), estrogen receptor (ER), and progesterone receptor (PR), traditional small-molecule inhibitors of therapeutic targets have little/no effect on patients suffering from TNBC in the clinical management. Even more alarmingly, the frequent relapse and metastasis of TNBC to distant organs (including lung, liver, brain, and bone) represents a severe clinical problem, as metastatic disease is usually a refractory event and is the primary cause of death for the vast majority of TNBC patients.
There are two main strategies for the treatment of metastatic TNBC: 1) arresting the highly aggressive circulating tumor cells (CTCs, the “seed” of metastasis) before dissemination to prevent the formation of metastases; 2) targeting the already formed metastases to kill metastatic tumor cells and inhibit tumor overgrowth. Thus, an ideal chemotherapeutic for metastatic TNBC should efficiently target and penetrate into TNBC or its metastatic sites. Typically, drug-loaded nanoparticles can target tumor site by size control and surface modification of targeting ligands toward tumor cell-overexpressing receptors. However, the specificity and targeting efficiency of tumor-targeting nanoparticles may be greatly challenged by tumor heterogeneity and off-target effects are prone to occur due to the molecular phenotype changes in TNBC. It is reported that platelets can be recruited and activated by tumor cells via intercellular adhesion molecules (ICAM) and aggregated around the tumor cells to form platelet–CTC complexes and help aggressive CTCs disseminate to distant organs in the intravasation/extravasation processes. Therefore, activated platelets have been considered with possession of tumor-homing, CTC-capturing, and metastasis-targeting abilities.
Recently, Professor Jiang Chen (corresponding author) Group of Fudan University developed a novel activated platelets-targeting micelle with controlled drug release to utilize activated platelets as an endogenous device for efficient drug delivery to both primary/metastatic tumors and significantly improves treatment outcome. In this work, a P-selectin (expressed on activated platelet surface) targeting peptide (PSN) is modified on a redox-responsive paclitaxel-loaded micelle (PSN-PEG-SS-PTX4 micelle) to utilize activated platelets as a “bridge” for interaction with cancer cells. The PSN-modified micelle can easily adhere to the surface of activated platelets and subsequently capture CTCs in blood circulation. PSN-PEG-SS-PTX4 micelle also exhibits enhanced primary TNBC/metastasis targeting and penetrating effect through binding with tumor infiltrating platelets. Subsequently, the micelle could be endocytosed by cancer cells and then disintegrated to release loaded chemotherapeutics under reductive cytosol conditions to induce strong anti-tumor effect. More importantly, PSN-PEG-SS-PTX4 micelle potently suppressed lung metastasis of TNBC and reduced incidence of distant liver metastasis. In conclusion, the activated platelet-targeting micelle with controlled drug release profile provides a promising strategy for the omnidirectional treatment of primary and metastatic cancers.
The research results were published in Advanced Functional Materials on Jan 9 entitled "Activated Platelets-Targeting Micelles with Controlled Drug Release for Effective Treatment of Primary and Metastatic Triple Negative Breast Cancer".
Full Text Link: https://doi.org/10.1002/adfm.201806620
Homepage of Jiang Chen Group: http://smartdds.fudan.edu.cn/jiangchen/index.aspx
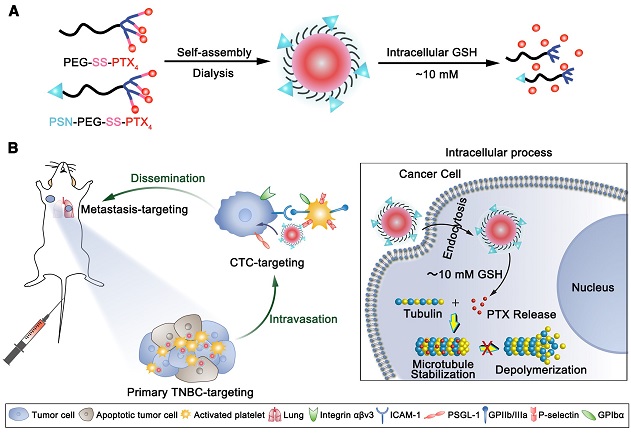
Figure 1. Schematic illustration of A) preparation and B) in vivo tumor targeting of PSN-PEG-SS-PTX4 micelles at different stages via activated platelets and intracellular process of the micelles.