
On December 6, 2018, Professor Jianxin Wang and Associate Professor Zhiqing Pang from Department of Pharmaceutics, School of Pharmacy, Fudan University jointly published a research paper entitled "Route to Rheumatoid Arthritis by Macrophage-Microvesicle-Coated Nanoparticles" in Nano Letters (IF=12.08). In this study, they presented a highly efficient method for extracting macrophage-derived vesicles and its application in targeting treatment of rheumatoid arthritis (RA).
The targeted delivery of therapeutics to the sites of RA has been a long-standing challenge. Inspired by the intrinsic inflammation-targeting capacity of macrophages, macrophage-derived microvesicle (MMV)-coated nanoparticle (MNP) was developed for targeting RA (Figure 1). MMV was efficiently produced through a novel method by applying cytochalasin B to relax the interaction between the cytoskeleton and membrane of macrophages, thus stimulating the secretion of MMV. The cell membrane deviation efficiency was increased by 50 fold compared with the conventional method. The proteomic profile of the MMV was analyzed by isobaric tags for relative and absolute quantitation. The results showed that the MMV membrane proteins were similar to those of macrophages, indicating that the bioactivity of MMV could be similar to that of macrophages. Poly (lactic-co-glycolic acid) (PLGA) nanoparticle (MNP) was subsequently coated with MMV, and the inflammation-mediated targeting capacity of the MNP was evaluated both in vitro and in vivo. The in vitro binding of MNP to inflamed HUVECs was significantly stronger than that of red blood cell membrane coated-nanoparticle (RNP). Compared with bare NP and RNP, MNP showed a significantly enhanced targeting effect in vivo in collagen-induced arthritis (CIA) mice. The outstanding targeting effect of the MNP could be attributed to the high binding affinity of Mac-1 and CD44. When tacrolimus, a model drug, was encapsulated in MNP, its effect on suppressing the progression of RA in mice was significantly improved. The present study demonstrates MMV as a promising and rich material to mimic macrophages, and that MNP is an efficient biomimetic vehicle for RA treatment.
Two doctoral students, Ruixiang Li and Yuwei He, from the School of Pharmacy, Fudan University are the co-first authors of the paper. Professor Jianxin Wang and Associate Professor Zhiqing Pang are the corresponding authors. The study was supported by the National Natural Science Foundation of China and Key Projects of Shanghai Science and Technology Commission.
DOI:10.1021/acs.nanolett.8b03439
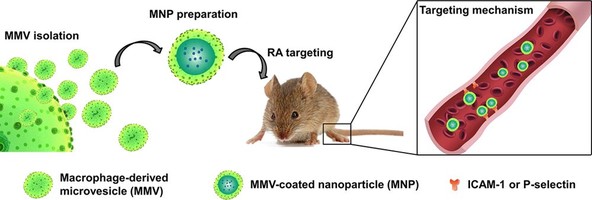
Figure 1 Schematic illustration of MMV-coated nanoparticle (MNP) targeting to the sites of RA through ICAM-1 or P-selectin adhesion.