
Alzheimer's disease (AD) is a progressive neurodegenerative disease. However, there is currently a lack of effective treatments to inhibit its progression, making it one of the most challenging brain diseases to treat clinically. Recent studies have shown that the phenotype and metabolic function of cells in the brain microenvironment are highly correlated with AD progression. It is significant to design treatment strategies based on the characteristics of the staged changes in the AD microenvironment.
In the previous work, Professor Chen Jiang's team from School of Pharmacy, Fudan University designed a peptide drug conjugate (PDC) for the early inflammatory microenvironment of AD. The PDC takes Nrf2, a key target for neurons to maintain their redox balance, and high levels of reactive oxygen species (ROS) in the microenvironment as the entry point and uses PDC as a tool to efficiently deliver Nrf2-activating peptides into the brain, thereby achieving neuroprotection, microglial transformation, and relief of neuroinflammation. Related research was published in Advanced Materials (https://onlinelibrary.wiley.com/doi/10.1002/adma.202100746). However, early diagnosis of AD is still a considerable challenge, and there is currently a lack of effective treatment strategies for most patients with mid- and late-stage AD.
Therefore, in this study, Professor Jiang's team constructed a PDC co-delivery platform based on the previous research on PDC. First, two PDCs coupled with glial cell regulatory drugs were synthesized and loaded on the surface of polydopamine (PDA) nanoparticles by electrostatic adsorption and covalent cross-linking. After intravenous injection, the delivery platform crossed the BBB through transcytosis, dissociated and released the two PDC-targeted glial cells in the inflammatory microenvironment in the brain. Hydroxychloroquine (HCQ) was targeted to microglia to activate immune response and restore energy metabolism. All-trans retinoic acid (ATRA) was targeted to astrocytes to promote transdifferentiation and reduce inflammation. This study abandoned the traditional neuron-centered brain disease treatment strategy and demonstrated a new method for regulating dormant glial cells to treat AD. At the same time, this study provides a new approach for PDC targeted delivery, providing new ideas for the multi-target treatment of diseases. The research was published in the latest issue of Advanced Materials in a paper titled "A Peptide-Drug Conjugate-Based Nanoplatform for Immunometabolic Activation and In Situ Nerve Regeneration in Advanced-Stage Alzheimer's Disease".

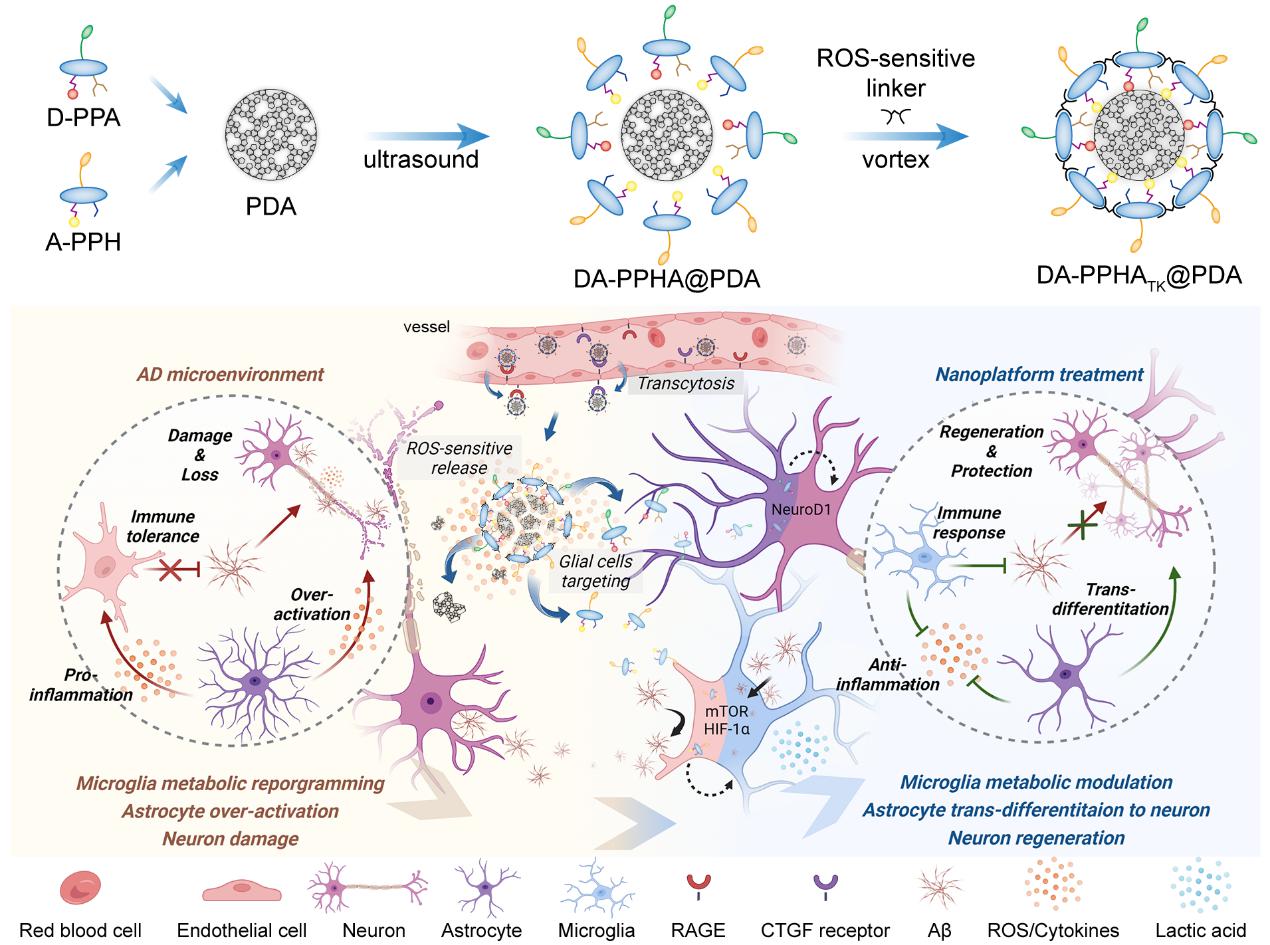
Schematic diagram of the preparation process of DA-PPHATK@PDA co-delivery system and its mechanism of modulating dormant glial cells
Article highlights
The delivery system has a spherical structure with a particle size of about 75 nm. In vitro and in vivo experiments have shown that DA-PPHATK@PDA can selectively target glial cells, activate the microglial metabolism-immune network, promote the degradation of toxic proteins, promote the activation of astrocyte stemness, and generate mature functional neurons in situ. In addition, it was unexpectedly discovered during the preparation of the delivery system that adding a cross-linking agent can induce the FRET effect, thereby diagnosing inflammation in the brain of AD transgenic mice.
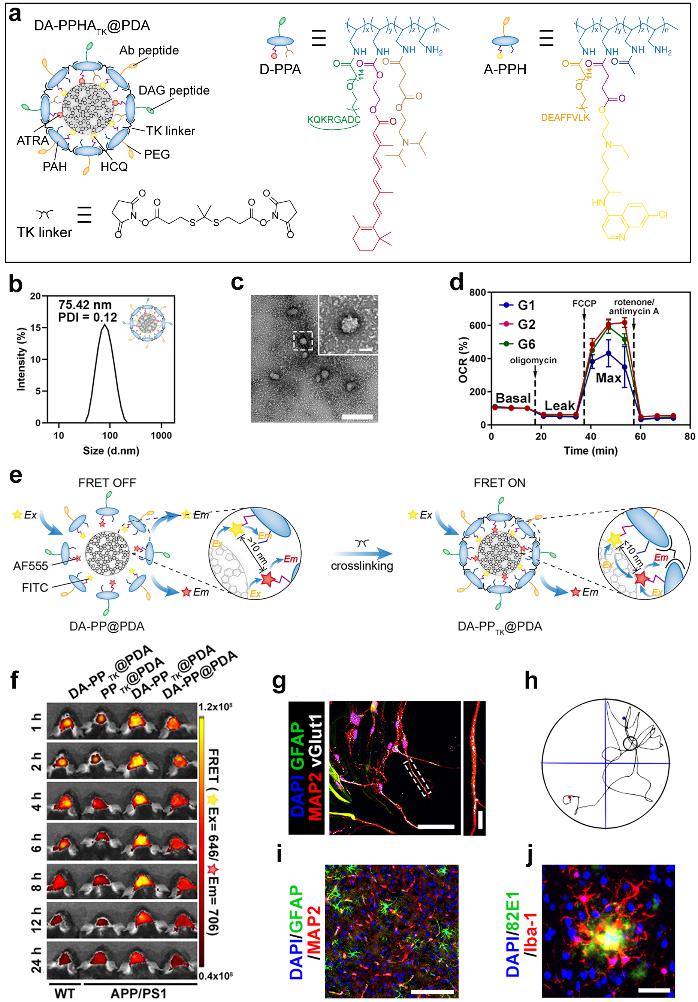 Schematic diagram of the DA-PPHATK@PDA structure and its regulatory effects in vivo and in vitro
Schematic diagram of the DA-PPHATK@PDA structure and its regulatory effects in vivo and in vitro
Summary and Outlook:
Currently, AD treatment still faces many challenges, which may be due to the complex and unclear pathogenesis, strong heterogeneity and the development of other diseases. More and more studies have shown that multi-target synergistic therapy is of great significance for AD. The PDC platform proposed in this study is universal. Through reasonable design, it can load multiple drugs in a certain proportion to achieve the purpose of multi-target treatment. In addition, the authors emphasized that the rational design of the brain microenvironment for the stage-by-stage changes in the progression of AD disease is conducive to its development in precision medicine. Finally, the cross-linking-induced FRET effect discovered by the authors is expected to become a favorable tool for diagnosing early brain inflammation in AD.
Peixin Liu, PhD student of 2019 from School of Pharmacy, Fudan University, is the first author, while Professor Chen Jiang is the corresponding author of the paper. The authors acknowledge the support from the National Natural Science Foundation of China (32030059, 82273865, and 82121002), Shanghai Municipal Science and Technology Major Project (Grant 2018SHZDZX01), and ZJLab.
Link: https://onlinelibrary.wiley.com/doi/10.1002/adma.202408729