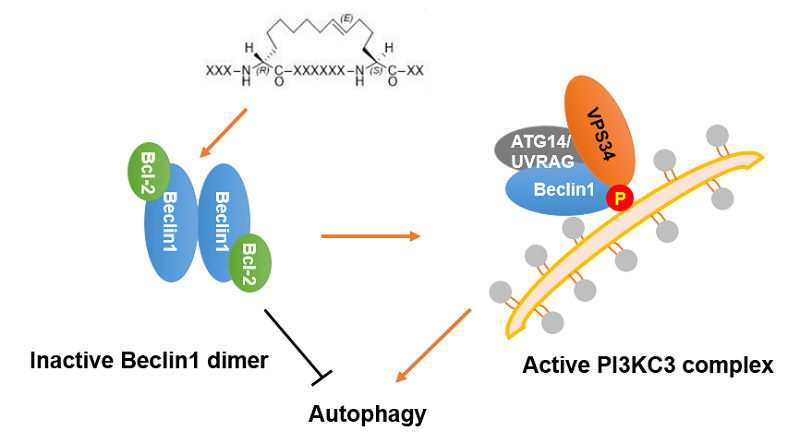
Beclin 1 is the scaffolding member of the Class III phosphatidylinositol-3-kinase (PI3KC3) complex and recruits two positive regulators Atg14L and UVRAG through its coiled-coil domain to upregulate PI3KC3 activity. Deficiency or dysfunction of Beclin 1 has been linked to human pathologies such as cancer and neurodegenerative diseases. Studies have shown that proteins such as epidermal growth factor receptor (EGFR) in non-small cell lung cancer and human epidermal growth factor 2 (HER2) in breast cancer can be degraded through the Beclin 1-mediated autophagy pathway. The homodimerization of Beclin 1 protein reduces autophagy levels in cells. Therefore, drug design targeting Beclin 1 may serve as an effective anti-proliferative strategy against EGFR- or HER2-driven cancers.
Prof. Renxiao Wang’s group of our school cooperated with Prof. Yanxiang Zhao’s group of Hong Kong Polytechnic University to target the protein-protein interaction formed by the homodimer of Beclin 1. Chemical structures with better binding affinity to the Beclin 1 coiled-coil domain were developed by staple scanning and sequence permutation based on the lead peptide, combined with molecular dynamics simulations and binding free energy calculations. Recently, the stage progress has been published online in the Journal of Medicinal Chemistry, an authorized journal in the field of medicinal chemistry issued by the American Chemical Society, entitled “Optimization of Beclin 1-Targeting Stapled Peptides by Staple Scanning Leads to Enhanced Antiproliferative Potency in Cancer Cells”. (Original link: https://doi.org/10.1021/acs.jmedchem.1c00870)
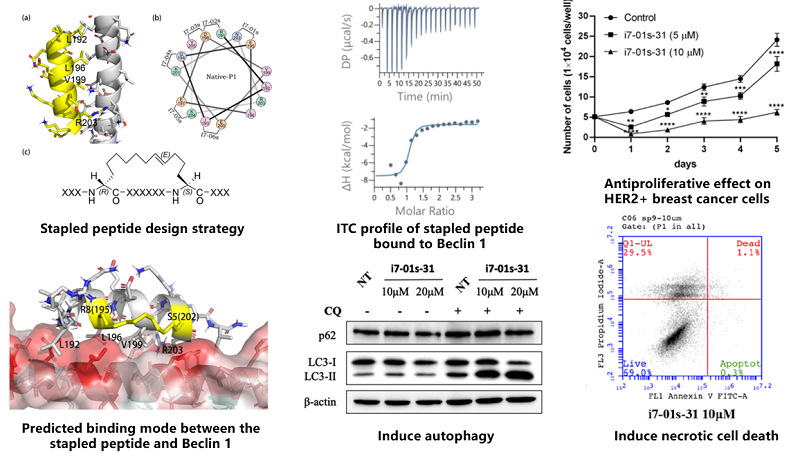
Based on the previous findings that hydrocarbon-stapled peptides attenuate Beclin 1 homodimerization and induce autophagy, this work proposes a staple scanning strategy combined with molecular dynamics simulations and binding free energy calculations to further improve the binding affinities between stapled peptides and Beclin 1. A subset of designed stapled peptides were synthesized and assayed, which exhibited comparable or stronger binding affinities (Kd ~ 100 nM) than the lead peptide. Structure-activity relationship analysis indicated that placing the hydrocarbon staple closer to the Beclin 1-peptide interface enhanced their binding affinity to the target by ~10- to 30-fold. The results of biological experiments confirmed that the optimized stapled peptides showed potent antiproliferative effects in cancer cells that overexpressed EGFR and HER2 by inducing necrotic cell death but not apoptosis. These stapled peptides targeting Beclin 1 offer new opportunities to develop drug candidates for the treatment of EGFR- or HER2-driven cancers.
Qifan Yang, a 2015 doctoral student at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Xianxiu Qiu, a doctoral student at the Hong Kong Polytechnic University, are the co-first authors of this collaborative paper. The School of Pharmacy, Fudan University is the affiliation of the last corresponding author. This work has been supported by the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (Grant No. 21661162003), the National Science Foundation for Distinguished Young Scholars of China (Grant No. 81725022), etc.
Design Strategy for Blocking Belin 1 Dimerization-Induced Autophagy by Stapled Peptides