
Fatty acid binding proteins (FABPs) are a family of tissue-specific expressed lipid chaperones that regulate fatty acid uptake and intracellular transport. Among the ten FABP subtypes, FABP4 and FABP5 are considered potential anti-inflammatory targets due to their significant roles in inflammation and metabolism-related signaling pathways. Studies have shown that upon FABP4 deletion, FABP5 expression is compensatorily upregulated, indicating functional overlap between the two proteins. Additionally, inhibiting FABP3 may lead to potential cardiotoxicity. Therefore, developing highly potent and selective dual FABP4/5 inhibitors is of great importance for treating inflammation-related diseases.
In recent years, Professor Yingxia Li’s group from School of Pharmaceutical Sciences, Fudan University, has discovered several structurally novel selective FABP4 and FABP4/5 inhibitors through virtual screening and structural optimization (Eur. J. Med. Chem. 2018; Bioorg. Med. Chem. 2019; Eur. J. Med. Chem. 2021; Eur. J. Med. Chem. 2023). Several selected compounds demonstrated significant anti-inflammatory activity both in vitro and in vivo.
Recently, in collaboration with Professor Wang Heyao’s group from Shanghai Institute of Materia Medica, they published a research paper titled “Discovery of Potent and Selective FABP4/5 Inhibitors with an Isoquinolone Scaffold as Potential Therapeutics for Inflammation-Related Diseases” in the Journal of Medicinal Chemistry. The paper reports a novel class of dual FABP4/5 inhibitors featuring an isoquinolone scaffold and systematically investigates their anti-inflammatory activity and mechanism of action.

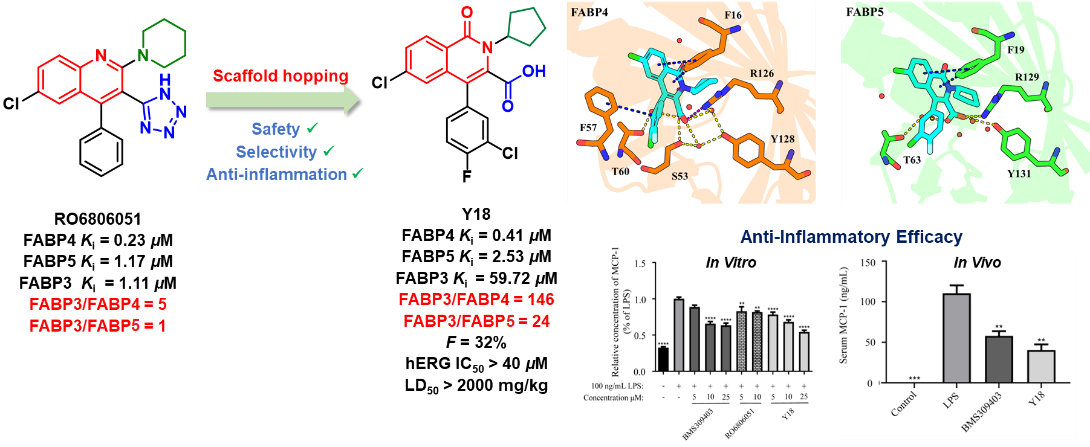
RO6806051, developed by Roche, is a dual FABP4/5 inhibitor but also exhibits strong FABP3 inhibition. Using RO6806051 as a lead compound and based on prior analysis of the differential binding modes of inhibitors to FABP4/5 versus FABP3, the research team employed a scaffold-hopping strategy to discover a novel class of dual FABP4/5 inhibitors containing an isoquinolone scaffold, achieving high selectivity over FABP3. Through structure-activity relationship studies, in vitro and in vivo anti-inflammatory activity evaluations, and drug-likeness assessments, compound Y18 was identified as a candidate. It exhibits potent inhibitory activity against FABP4 and FABP5 with Ki values of 0.41 μM and 2.53 μM, respectively, and a Ki value of 59.72 μM for FABP3. This selectivity profile is significantly superior to that of RO6806051 (FABP3 Ki/FABP4 Ki = 5, FABP3 Ki/FABP5 Ki = 1). Y18 demonstrates favorable pharmacokinetic properties with an oral bioavailability of 32%. Oral administration of Y18 alleviated LPS-induced liver injury. Furthermore, the compound exhibits an excellent safety profile: weak hERG inhibition (IC50 > 40 μM), low cytotoxicity (IC50 > 40 μM), and high median lethal dose (> 2000 mg/kg). In summary, Y18 is a potent, highly selective, and orally active dual FABP4/5 inhibitor with potential for treating inflammation-related diseases.
The co-first authors of this paper are Dr. Yulong He and Yuqi Chen from the Department of Medicinal Chemistry, School of Pharmaceutical Sciences, Fudan University, and Shunyi Li and Yiyang Xu from Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The corresponding authors are Professor Yingxia Li and Dr. Zonglong Chen from School of Pharmaceutical Sciences, Fudan University, and Professor Heyao Wang from Shanghai Institute of Materia Medica, Chinese Academy of Sciences. This research was supported by grants from the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences.
Link: https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c01256