
July 31, 2025—Prof. Lu Zhou’s lab from School of Pharmaceutical Sciences, Fudan University, collaborating with Prof. Changliang Shan’s group from Nankai University, reports the development of a novel series of uncompetitive inhibitors targeting 6-phosphogluconate dehydrogenase (6PGD), a key enzyme in tumor metabolism, for potential cancer therapeutics. This work has been published online in the Journal of Medicinal Chemistry entitled as “Discovery of N-(1,3,4-Thiadiazol-2-yl)amide Derivatives as Uncompetitive Inhibitors of 6-Phosphogluconate Dehydrogenase”.

Metabolic reprogramming is a hallmark of cancer cells, driving tumorigenesis, progression, and therapy resistance. The pentose phosphate pathway (PPP), a critical glucose metabolic pathway, includes 6-phosphogluconate dehydrogenase (6PGD), an NADP+-dependent oxygenase that converts 6-phosphogluconate (6PG) to ribulose-5-phosphate (Ru-5-P) with the reduction of NADP+ to NADPH. NADPH is an important antioxidant involved in scavenging reactive oxygen species (ROS), and Ru-5-P serves as an important precursor for nucleic acid synthesis and DNA repair under oxidative stress. Dysregulation of 6PGD is associated with tumor growth, progression, and resistance to drugs and radiation, making it a promising therapeutic target. However, potent 6PGD inhibitors remain limited.
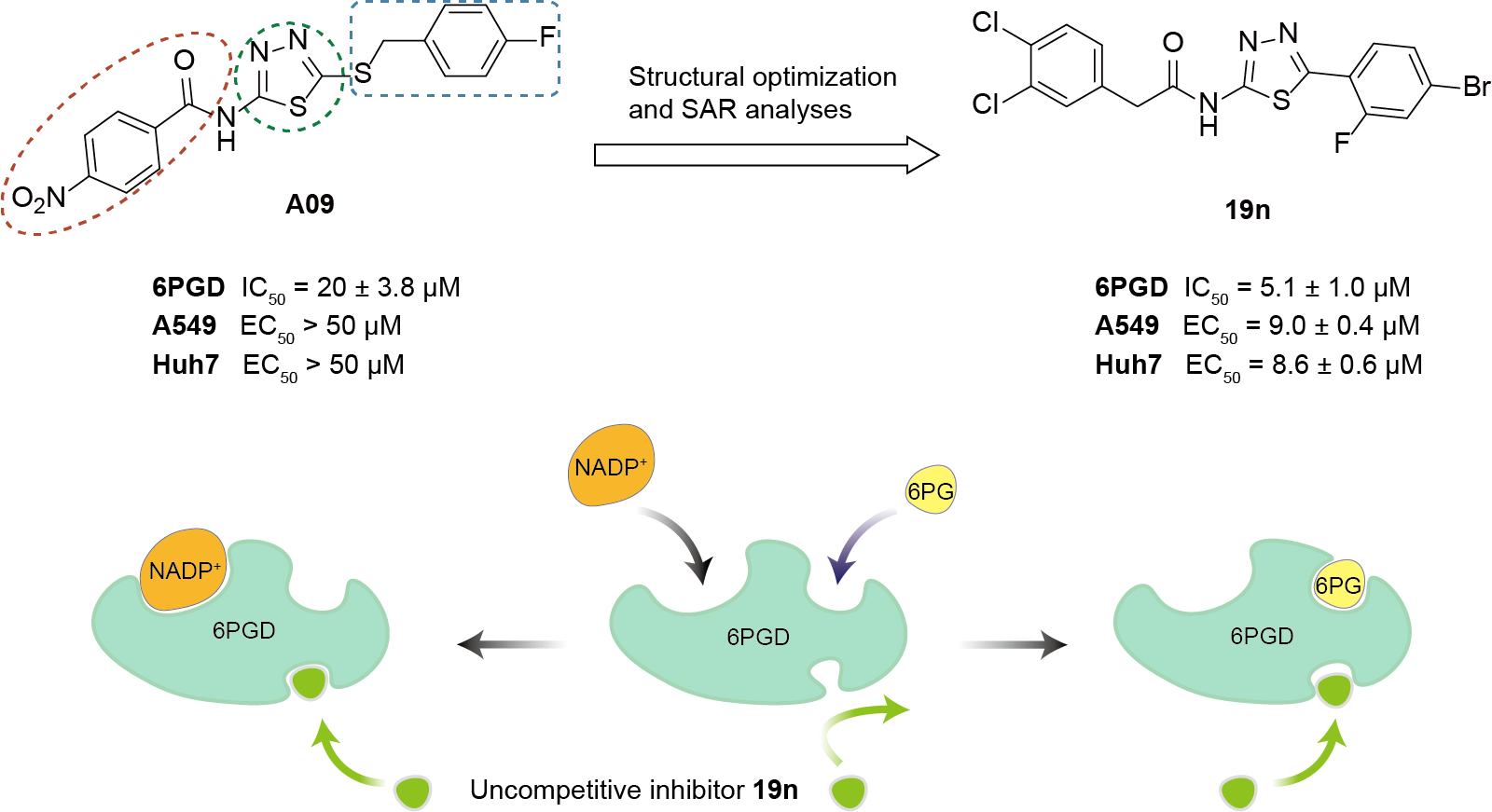
This work identified a series of N-(1,3,4-thiadiazol-2-yl)amide derivatives as uncompetitive 6PGD inhibitors, through high-throughput screening and structure−activity relationship analysis. Compound 19n emerged as a potent inhibitor, disrupting 6PGD oligomerization in a substrate-dependent manner and suppressing the proliferation of A549 and Huh7 cancer cells. This work provides a new scaffold compound 19n which expands the chemical space of 6PGD inhibitors with a novel mechanism and is worthy of further study.
Rong Zhang (School of Pharmaceutical Sciences, Fudan University) and Mingming Sun (Nankai University) are the co-first authors. The corresponding authors are Prof. Lu Zhou from the School of Pharmaceutical Sciences, Fudan University, and Prof. Changliang Shan from Nankai University. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Shanghai Municipal Committee of Science and Technology, and the Natural Science Foundation of Tianjin. Additionally, National Facility for Protein Science in Shanghai (NFPS), Zhangjiang Lab, China, provided the technical support and assistance in data collection and analysis.
The original link: https://doi.org/10.1021/acs.jmedchem.4c03215