
On October 4, 2025, a joint team led by Professor Cong Li from Fudan University School of Pharmaceutical Sciences, Young Investigator Xihui Gao from the School of Basic Medical Sciences, and Young Investigator Cong Wang from the School of Pharmaceutical Sciences published a landmark study in the internationally renowned journal Cell Reports. Aiming at the dilemma that existing treatments and conventional immunotherapies have limited benefits for glioblastoma (GBM)—the most common and fatal primary brain tumor in adults—the team developed a "programmed regulatory adoptive macrophage phenotype technology" and successfully created phenotype-programmed macrophages, providing an innovative solution to break through GBM treatment bottlenecks.
GBM treatment has long faced four core challenges: immunosuppressive microenvironment, tumor heterogeneity, difficulty in crossing the blood-brain barrier, and low immunogenicity of neoantigens. These issues result in surgery, radiotherapy, chemotherapy and existing immunotherapies failing to significantly extend patients' survival. Although tumor-associated macrophages (TAMs) account for 30%-50% of total GBM cells, traditional strategies have been unable to efficiently utilize their therapeutic potential.
A key finding of the team is that anti-inflammatory phenotype macrophages are more likely to cross the blood-brain barrier than pro-inflammatory phenotype ones. Based on this, researchers constructed a polymer containing reactive oxygen species (ROS)-responsive STING agonists, and co-incubated it with anti-inflammatory phenotype macrophages to prepare engineered M2-like macrophages (eM2-Mφs). These cells can achieve "programmed action": after intravenous injection, they efficiently cross the blood-brain barrier and infiltrate tumors in the anti-inflammatory phenotype; then, in response to high concentrations of ROS in the tumor microenvironment, they release STING agonists and convert to the pro-inflammatory phenotype, releasing cytokines to reverse the immunosuppressive microenvironment.
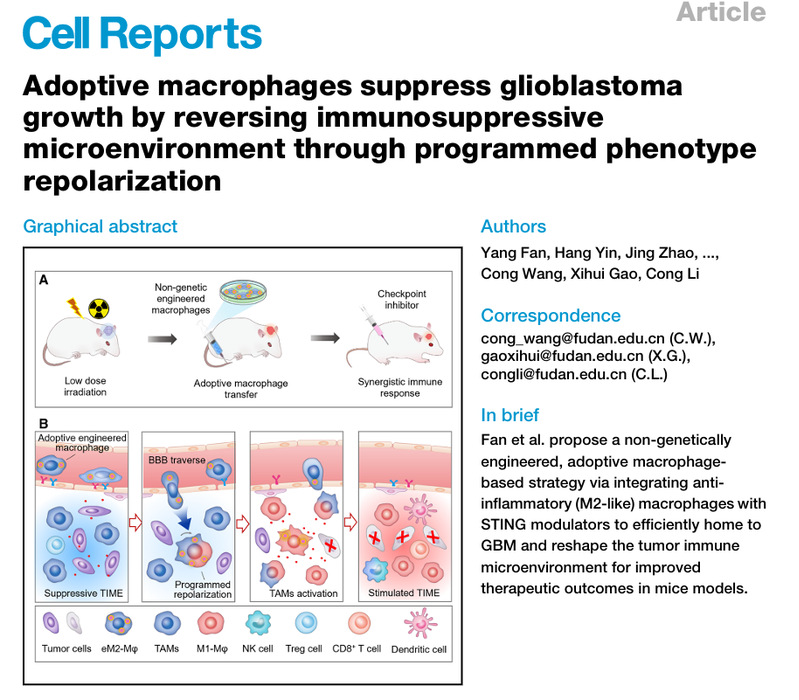
Experiments on GBM mouse models showed that eM2-Mφs alone can significantly inhibit tumor growth. And when combined with low-dose radiotherapy or immune checkpoint inhibitors, they can greatly extend the survival period of mice. The co-first authors are doctoral students Yang Fan, Hang Yin, Jing Zhao and Bolin Yao from School of Pharmaceutical Sciences, Fudan University. The co-corresponding authors are Professor Cong Li, Young Investigator Xihui Gao from School of Basic Medical Sciences, and Young Investigator Cong Wang from the School of Pharmaceutical Sciences. The research was supported by the National Key R&D Program, the National Natural Science Foundation of China and other projects.
Original link: https://www.sciencedirect.com/science/article/pii/S2211124725011210