
Maintaining intracellular oxygen homeostasis is fundamental to normal physiological function. Hypoxia-inducible factor-1α (HIF-1α) is a master regulator of cellular responses to oxygen, participating in a wide range of biological processes. Activation of the HIF-1α pathway is commonly used to treat anemia, whereas inhibiting this pathway represents a potential anti-tumor strategy. Although six HIF-1α–activating drugs have been approved, direct HIF-1α inhibitors remain unapproved and largely confined to clinical trials. This bottleneck may arise because HIF-1α regulates numerous pathways; consequently, direct inhibition of HIF-1α can lead to side effects and safety concerns. In this context, prior work by Academician Guoqiang Chen’s team identified CBX4-mediated SUMOylation enhances HIF-1α activity and contributes to tumor progression (Cancer Cell, 2014). Thus, suppressing CBX4-driven hyperactivation of HIF-1α could effectively inhibit tumor growth and mitigate the adverse effects associated with direct HIF-1α inhibitors.
A collaborative team led by Prof. Lu Zhou (School of Pharmaceutical Sciences, Fudan University), together with Academician Guoqiang Chen (Shanghai Jiao Tong University School of Medicine) and the clinical team of Academician Jia Fan / Dr. Jiabin Cai (Zhongshan Hospital, Fudan University), now reports a previously unrecognized inhibitory checkpoint: acetoacetylation of CBX4 at lysine 106 (CBX4 K106acac) suppresses CBX4’s SUMO E3 ligase activity and thereby reduces HIF-1α SUMOylation and transcriptional activity. The study, entitled “CBX4 acetoacetylation as an inhibitory mechanism of HIF-1α activity,” is published in Cell Chemical Biology.
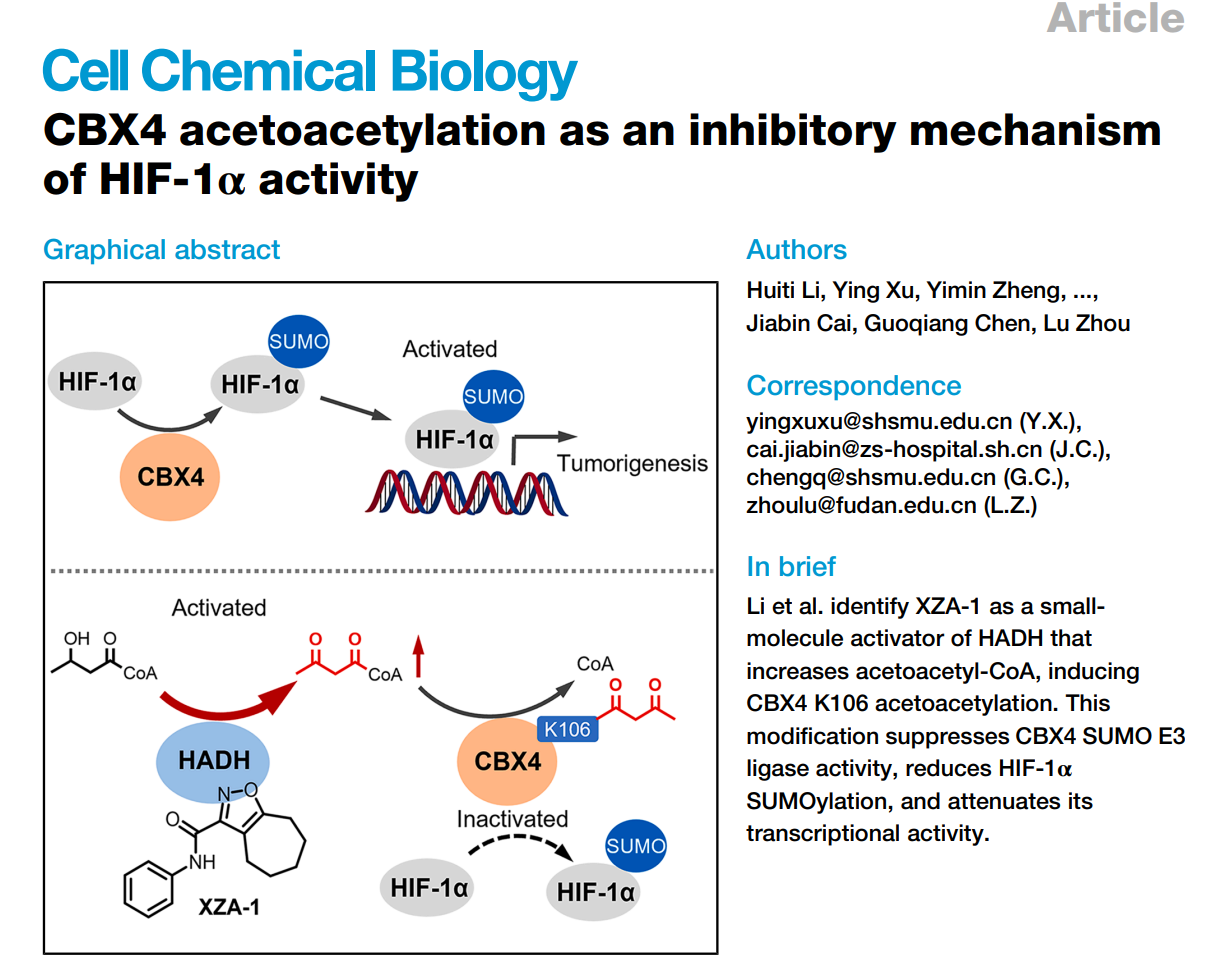
The collaborative team established a novel dual-luciferase reporter assay for phenotypic high-throughput screening, and identified a series of cycloheptane isoxazole compounds that suppress CBX4-enhanced HIF-1α transcriptional activity. Through structure optimization and chemical synthesis, they obtained a potent lead compound, XZA-1, and a photoaffinity probe, XZA-2. Using affinity-based protein profiling (ABPP) coupled to LC–MS/MS, the molecular target was identified as 3-hydroxyacyl-CoA dehydrogenase (HADH). Target engagement and mechanism were validated by knockdown/knock-in, co-immunoprecipitation, metabolomics, proteomic mass spectrometry, and molecular docking. The results reveal that these compounds activate HADH enzymatic activity, elevate acetoacetyl-CoA levels, increase acetoacetylation of CBX4 at Lys106 (CBX4 K106acac), thereby suppressing CBX4 SUMO E3 ligase activity and ultimately inhibiting HIF-1α SUMOylation and transcriptional output. Furthermore, clinical data indicate that CBX4 K106acac is a potential prognostic biomarker in hepatocellular carcinoma. Collectively, these findings uncover a potential new link between cancer metabolism and the tumor microenvironment and open a new avenue for anticancer drug development.
Co–first authors are Huiti Li (Postdoctoral Fellow, School of Pharmaceutical Sciences, Fudan University), Ying Xu (Associate Researcher, Shanghai Jiao Tong University School of Medicine), Yimin Zheng (Ph.D., Zhongshan Hospital, Fudan University), Zian Xue (M.S., School of Pharmaceutical Sciences, Fudan University), and Qingqing Li (Ph.D., Shanghai Jiao Tong University School of Medicine). The corresponding authors areAssociate ResearcherYing Xu, Academician Guoqiang Chen, Associate Chief Physician Jiabin Cai, and Professor Lu Zhou. The study also received strong support from Researcher He Huang (Institute of Metabolism and Integrative Biology, Fudan University), Researcher Jin Li (School of Life Sciences, Fudan University), and Researcher Minjia Tan (Shanghai Institute of Materia Medica, Chinese Academy of Sciences). This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Shanghai Municipal Science and Technology Commission Basic Research Special Zone Program.
Original article link:https://pubmed.ncbi.nlm.nih.gov/41045931/