
Recently, a team led by Prof. Lu Zhou from School of Pharmaceutical Sciences at Fudan University, together with collaborators Prof. Liang Zhang (Shanghai Jiao Tong University), Prof. Meiqing Feng (Fudan University), and Prof. Hongkai Bi (Nanjing Medical University/Hainan Medical University), reported a study in the Journal of Medicinal Chemistry entitled "Discovery of 1,3,4-Thiadiazole Sulfonamide-Based Potent Inhibitors against the Unsaturated Fatty Acid Synthase FabX of Helicobacter pylori". The study reports a series of potent, novel FabX inhibitors, advancing FabX as a promising therapeutic target for anti-H. pylori therapy.
H. pylori is a common gastric pathogen linked to gastritis and gastric cancer, infecting nearly half of the global population. Current treatments rely on broad-spectrum antibiotics, which can disrupt the microbiome and face rising resistance. Unsaturated fatty acids are essential for maintaining bacterial membrane integrity and regulating membrane fluidity. In H. pylori, these fatty acids are synthesized by FabX, a non-canonical bifunctional dehydrogenase/isomerase uniquely expressed in H. pylori and crucial for bacterial growth and gastric colonization. Targeting FabX offers a precision approach that could reduce off-target effects on beneficial bacteria.
In this study, high-throughput screening identified a hit compound, P61G11, featuring a 1,3,4-thiadiazole sulfonamide scaffold. Its inhibitory potency and binding affinity toward FabX were validated experimentally. X-ray crystallography reveals that the compound binds to the FMN catalytic site of FabX, blocking entry of the acyl-ACP phosphopantetheine arm and oxygen, thereby suppressing the catalytic activity. Through structure-guided optimization, the team advanced compound 47, which showed strong enzymatic inhibition (IC₅₀ = 0.128 ± 0.002 μM) and robust anti-H. pylori activity (MIC = 0.5 μg/mL) when combined with an outer-membrane permeabilizer (e.g., EDTA) or efflux-pump inhibitor (e.g., PAβN). Notably, the compound showed negligible activity against non-H. pylori strains, suggesting a promising narrow-spectrum profile. These findings open a path toward new H. pylori therapies that are both selective and effective.
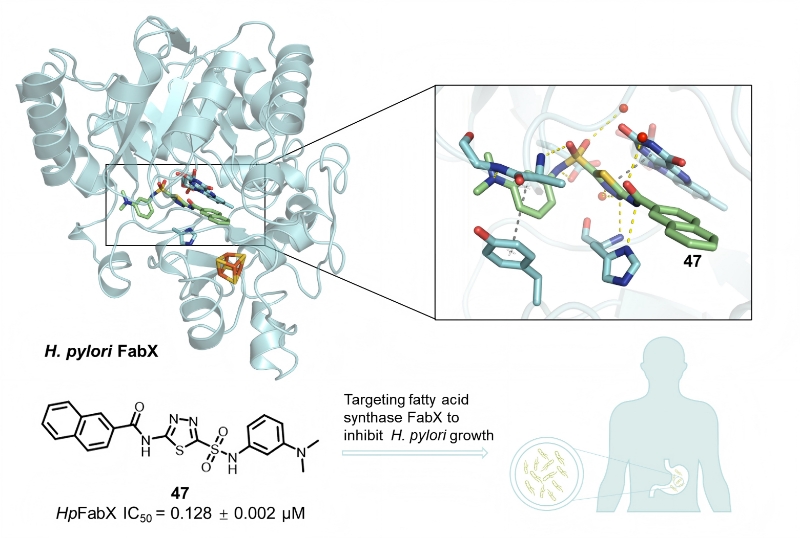
Figure 1Structure, activity, and binding mode of compound 47 in complex with FabX.
Xiaoxue Ruan (School of Pharmaceutical Sciences, Fudan University), Lin Zhang (Shanghai Jiao Tong University), and Linlin Dong (Nanjing Medical University) are co–first authors. Prof. Lu Zhou (School of Pharmaceutical Sciences, Fudan University), Prof. Liang Zhang (Shanghai Jiao Tong University), Prof. Meiqing Feng (School of Pharmaceutical Sciences, Fudan University), and Prof. Hongkai Bi (Nanjing Medical University/Hainan Medical University) are the corresponding authors. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Shanghai Science and Technology Commission, with additional platform support from the National Facility for Protein Science in Shanghai.
Full Article: https://doi.org/10.1021/acs.jmedchem.5c00654