
Diquat, a highly toxic herbicide, can induce acute kidney injury, leading to an extremely high mortality rate. Currently, for the treatment of poisoning represented by diquat, clinical methods mainly rely on blood purification technologies. However, these technologies are unable to effectively eliminate toxins accumulated in tissues and fail to prevent multiple organ failure caused by oxidative stress and inflammatory responses. To address this challenge, this study proposes a bioinspired precise detoxification strategy and develops a rotaxane nanoscavenger system escorted by engineered macrophages. This system enables precise recognition, capture, and detoxification of diquat, while alleviating oxidative damage to organs and improving the survival rate of poisoned mice.
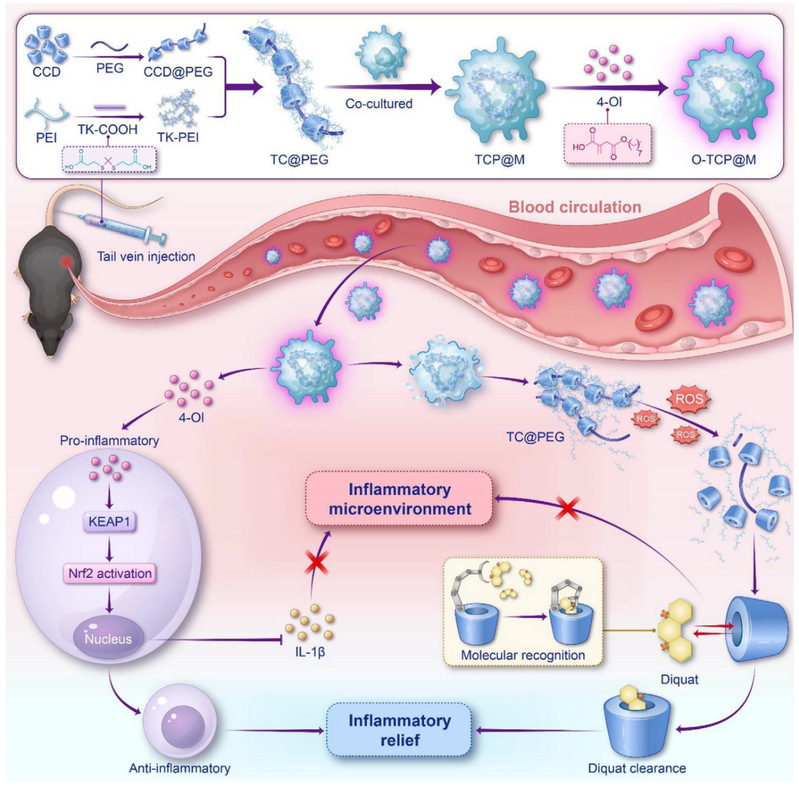
The demand-oriented detoxification strategy proposed in this study aims to solve the key unmet needs in poisoning management. Through the design of carboxymethyl α-cyclodextrin (CCD) molecules assisted by complementary toxin structures, artificial intelligence (AI), and molecular simulations, the system exhibits excellent toxin recognition and neutralization capabilities. Furthermore, a rotaxane-based architecture integrates CCD into a polyethylene glycol (PEG) carrier; after stabilization with a reactive oxygen species (ROS)-responsive polymer, the complex is loaded into macrophages, demonstrating significant targeting ability to renal inflammatory sites. Meanwhile, the system is loaded with 4-octylcinnamate (4-OI) to activate the body’s nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant pathway, reducing inflammatory levels and ROS-induced damage. This dual-pronged approach achieves a paradigm shift from passive toxin clearance to active toxin neutralization at the tissue level.
The persistently high mortality rate of diquat poisoning is primarily attributed to its unique dipyridinium cationic structure. This structure allows diquat to specifically accumulate in the kidneys, bind to renal tubular epithelial cells, induce excessive production of ROS, and subsequently trigger oxidative stress and inflammatory responses, ultimately leading to renal failure. Traditional therapeutic approaches such as hemodialysis cannot effectively remove diquat that has accumulated in renal tissues. Therefore, there is an urgent need to develop a novel therapy that can actively target toxin-accumulating tissues and break the pathological feedback loop.
In this study, an antidote based on the principle of charge neutralization—polysubstituted carboxymethyl α-cyclodextrin (CCD)—was designed and synthesized. Through molecular simulation calculations and AI screening, it was confirmed that CCD can precisely recognize and capture diquat molecules while inhibiting ROS generation. To enhance renal targeting, negatively charged CCD was threaded onto PEG chains to form a "molecular necklace"-like pseudopolyrotaxane structure. This structure was coated and stabilized using a cationic polymer that undergoes ROS-responsive cleavage, and the resulting complex was encapsulated in macrophages. Leveraging the inherent inflammatory chemotaxis of macrophages, active targeted delivery to the kidneys was achieved. Additionally, 4-OI was incorporated into this biomimetic delivery system to activate the Nrf2 antioxidant pathway, synergistically breaking the ROS-inflammation vicious cycle.
Proposal of an active detoxification strategy: This strategy enables precise recognition and active capture of toxins, breaking the limitations of traditional passive detoxification therapies (e.g., hemodialysis). It opens up a new avenue for poisoning treatment, shifting from passive toxin clearance to active "toxin hunting".
Precise molecular design of the antidote: Through computational screening, CCD was identified as an ideal host molecule. It neutralizes the positive charge of diquat via multivalent carboxylate interactions and matches diquat in molecular structure, enabling precise toxin capture.
Multifunctional biomimetic delivery system: By combining the advantages of the rotaxane structure and macrophages, the system overcomes renal targeting barriers and enables stimuli-responsive release triggered by the pathological microenvironment, achieving precise delivery to toxin-accumulating tissues. Moreover, it activates the Nrf2 antioxidant pathway, addressing both the symptoms and root causes of poisoning to realize synergistic detoxification.
In conclusion, this study successfully developed a rotaxane nanoscavenger system escorted by engineered macrophages, providing a novel solution for precise detoxification (represented by diquat poisoning). The system not only achieves efficient capture and detoxification of diquat but also effectively alleviates renal oxidative damage and inflammatory responses, improving the survival rate of poisoned mice. This innovative strategy, which integrates supramolecular design, functional topological engineering, and cell-driven biodistribution, sets a precedent for the transformation from passive symptom management to active mechanism-based intervention in the field of emergency toxicology and is expected to provide a reference for the treatment of other types of poisoning.
Relevant research findings were published in Advanced Materials (a CAS JCR Q1 journal) under the title Engineered-Macrophage-Escorted Rotaxane Nanoscavengers for Precise Diquat Detoxification. The corresponding authors of the paper include Tao Sun (Department of Pharmaceutics, School of Pharmaceutical Sciences, Fudan University), Keyu Sun and Zichen Xie (Emergency Department, Minhang Hospital Affiliated to Fudan University), and Jiaqi Wang (University of Hong Kong), among others. This research work was supported by projects such as the General Program of the National Natural Science Foundation of China (NSFC) and the International Cooperation Program of the Shanghai Science and Technology Commission.
Original article link: https://advanced.onlinelibrary.wiley.com/doi/10.1002/adma.202506466