
Microtubules constitute a major component of the cellular cytoskeleton. Malignant tumor cells exhibit robust proliferation and highly active mitosis, making microtubules one of the most successful targets for anticancer therapy. Despite the availability of several marketed microtubule-targeting agents (MTAs), the rapid development of resistance has led to diminishing clinical efficacy over prolonged treatment. Katanin, a microtubule-severing protein, hydrolyzes ATP to promote microtubule depolymerization. Therefore, dual-target compounds that simultaneously affect both tubulin and katanin may enhance microtubule dynamics disruption and exhibit potent anticancer effects. This unique mechanism holds promise in overcoming resistance issues currently observed with microtubule-targeting drugs.
In recent years, Professor Yang Wang's research group at the School of Pharmacy, Fudan University, has reported a variety of novel tubulin polymerization inhibitors and systematically elucidated their structure-activity relationships (J. Med. Chem.2016, 59, 10329; Bioorg. Med. Chem.2017, 25, 6623; Eur. J. Med. Chem. 2018, 144, 817; Eur. J. Med. Chem. 2019, 178, 177; Eur. J. Med. Chem. 2020, 197, 112323; Eur. J. Med. Chem.2021, 214, 113256; Eur. J. Med. Chem.2021, 221, 113531; Bioorg. Chem.2021, 115, 105239; Eur. J. Med. Chem.2022, 236, 114344; Bioorg. Chem.2022, 128, 106112; Bioorg. Med. Chem.2023, 92, 117437). Multiple optimal compounds have been demonstrated significant in vitro and in vivo anticancer activities along with favorable safety profiles.
Recently, Professor Yang Wang's group published a research paper entitled "Discovery of Novel Diaryl-Substituted Fused Heterocycles Targeting Katanin and Tubulin with Potent Antitumor and Antimultidrug Resistance Efficacy" in the authoritative medicinal chemistry journal, Journal of Medicinal Chemistry. The study reports the discovery of a series of novel diaryl-substituted fused heterocycles targeting katanin and tubulin, demonstrating strong efficacy against tumors and multidrug resistance, while elucidating their mechanism of action.
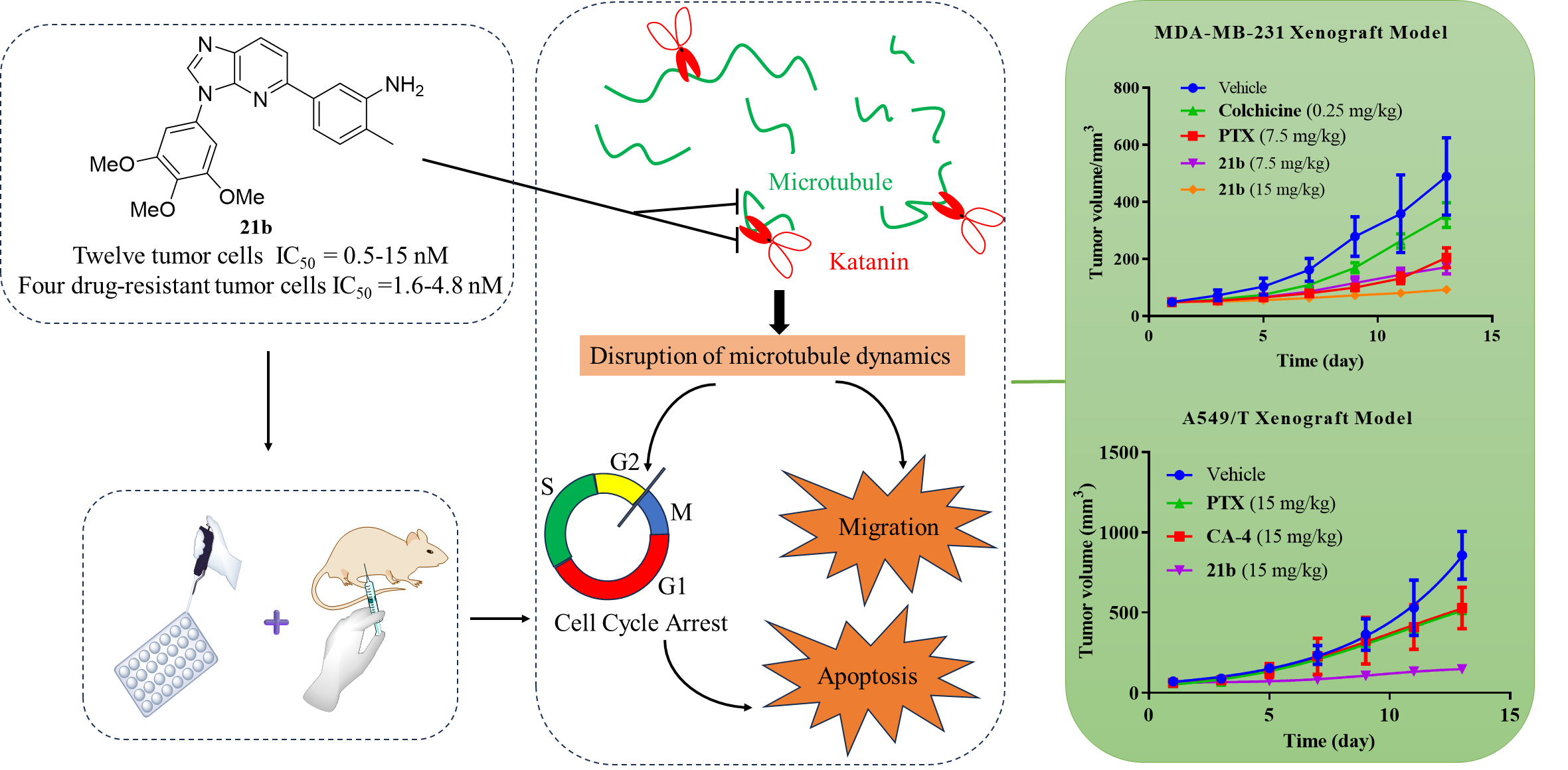
Based on the previous research, the group employed rational design, phenotypic screening, and iterative optimization to identify several highly potent antitumor compounds. Subsequent studies on drug-like evaluation and detailed biological activities led to the discovery of a novel 3H-imidazo-[4,5-b]pyridine-based dual katanin and tubulin regulator represented by compound 21b. Mechanistic investigations revealed that optimized compound 21b exhibited excellent dual targeting of katanin and tubulin, disrupting the tumor cell microtubule network, inducing G2/M cell cycle arrest, and subsequently promoting apoptosis. Compound 21b also demonstrated strong anti-proliferative effects (IC50 = 0.5-15 nM) against 12 tumor cell lines and 4 multidrug-resistant tumor cell lines, inhibited colony formation and angiogenesis in vitro, and downregulated metastasis-associated protein expression. Acute toxicity studies indicated an LD50 value of 150 mg/kg for 21b, indicating favorable safety profiles. In breast cancer (MDA-MB-231) xenograft tumor models, administration of 21b at doses of 7.5 and 15 mg/kg significantly inhibited tumor growth without obvious toxic effects. In a paclitaxel-resistant lung cancer (A549/T) xenograft model, 21b overcame clinical relevance by exhibiting excellent single-agent in vivo antitumor efficacy and safety, suggesting promising prospects for further development and application.
Fuhao Jiang (Ph.D. candidate, Class of 2021) and Min Yu (Ph.D. candidate, Class of 2023) from the Department of Medicinal Chemistry, School of Pharmacy, Fudan University, are co-first authors of this study. Professor Yang Wang is the corresponding author. This research is supported by grants from the National Natural Science Foundation of China.
Original link: https://doi.org/10.1021/acs.jmedchem.4c00878