
Recently, the research group led by Prof. Chen Jiang has developed a versatile drug delivery system that can trigger transcytosis. The delivery system can efficiently deliver chemotherapy drugs to the deep tumor, regulate the microenvironment, and activate the anti-PDAC immune response. The study was published online in Advanced Functional Materials, named Transcytosis Mediated Deep Tumor Penetration for Enhanced Chemotherapy and Immune Activation of Pancreatic Cancer.

The hyperproliferative tumor stroma of pancreatic ductal adenocarcinoma (PDAC) severely limits drug permeation and constructs an immunosuppressive microenvironment, causing resistance to chemotherapy and immunotherapy. Traditional nanomedicine mainly focuses on manipulating nanoparticles’ size or electrical characteristics to penetrate deep PDAC through the paracellular pathway, but the transcellular pathway is often ignored. Transcytosis has become an important way of transporting macromolecular nutrients in tumors, as the conventional transport routes cannot sustain their exuberant cellular metabolic demands. Therefore, the rational design of nanomedicines that can trigger transcytosis would provide a strategy to bypass the long-term bottleneck of nanomedicine delivery in anti-PDAC therapy.
As a typical cold tumor, PDAC confronts inadequate immune cell activation and infiltration, which limit the immune response against PDAC. The stimulator of interferon genes (STING) pathway has arisen as a promising target for energizing anti-tumor immune responses. It simultaneously provides new alternations for immunotherapeutic strategies in PDAC treatment. A lately reported polymer with cyclic tertiary amine structures (PHMI) exhibited STING-dependent immune activation ability. Meanwhile, as the primary driver of desmoplasia in PDAC, FAK acts as a critical molecular fulcrum in reshaping the tumor microenvironment and affecting the infiltration of both drugs and immune cells into the deep tumor. Other than STING activation, PHMI can also sense the mild acidity in tumors, and realize the hydrophobicity to hydrophilicity transformation. This feature energizes PHMI as an advanced FAK inhibitor delivery carrier and anti-tumor immune response stimulator synergistically.
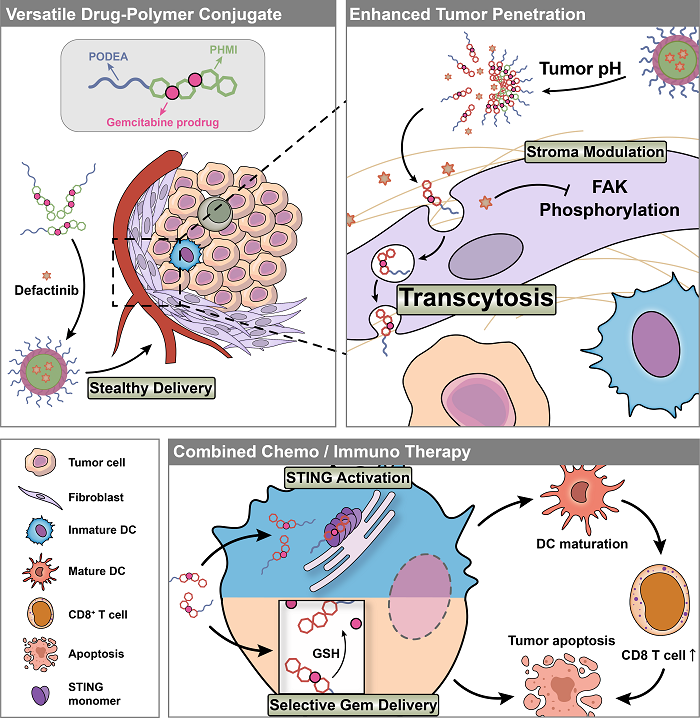
Herein, a multifunctional drug-polymer conjugate PODEA-Gem-HMI which can trigger transcytosis, was designed, prepared, and assembled into dual-responsive nanoparticles for the codelivery of chemotherapeutic drug gemcitabine and selective FAK inhibitor defactinib. PODEA is a zwitterion polymer showing high phospholipid affinity but not to proteins, which guarantees prolonged systematic circulation. Upon reaching the tumor tissue, the nanoparticle would disintegrate and release defactinib in the TME as sensing the specific mild acidity. And the unique structure of PODEA enables the reversible binding between the conjugates and cell membranes and induces adsorption-mediated transcytosis, promoting tumor extravasation and penetration. To avoid gemcitabine being taken up by non-tumor cells and causing unwanted side effects, we conjugated gemcitabine to the polymer backbone with a reduction-responsive disulfide bond. When the conjugate is internalized by tumor cells, the overexpressed intracellular GSH can destroy the disulfide bonds and release gemcitabine to restrain tumor proliferation. Meanwhile, after cellular uptake, PHMI would activate the STING pathway and promote cytokine expression.
In short, this versatile drug-polymer conjugate constituted multifunctional nanoparticle can prerelease defactinib triggered by mild acidity in the TME, transport gemcitabine to the deep tumor through transcytosis, and activate the STING pathway for TME modulation, chemotherapy promotion, and spontaneous immune activation, providing a promising combined strategy for PDAC treatment.
HongyiChen, the PhD fellow from the School of Pharmacy, Fudan University, is the first author. Professor Chen Jiang is the corresponding author, and Associate Professor Tao Sun is the co-corresponding author of this paper. The work was supported by grants from the National Natural Science Foundation of China, Key Projects of Shanghai Science Foundation, Shanghai Municipal Science and Technology Major Project, and ZJLab.
For more information: https://doi.org/10.1002/adfm.202214937