
In recent years, the tumor immune regulatory function of adenosine A2A receptor (A2AR) has attracted much attention, and A2AR has been identified as an emerging potential target for cancer immunotherapy. In the tumor microenvironment (TME), hypoxia upregulates the expression of CD39 and CD73 through hypoxia-inducible factor 1 (HIF-1) and other cytokines, such as transforming growth factor-β (TGF-β), which increases the enzymatic hydrolysis of ATP, resulting in a greatly increased level of extracellular adenosine. Adenosine accumulation prevents tumors from the attack of the immune system. The immunosuppression responses generated by adenosine via A2AR include: inhibiting the cytotoxicity of CD8+ T cells, inhibiting the killing ability of natural killer (NK) cells, enhancing the immunosuppressive activity of regulatory T cells (Treg), and so on.Therefore, the discovery of novel small molecule A2AR antagonists is of great significance for the development of tumor immunotherapy.
One of the research directions of Prof. Yonghui Wang and Assoc Prof Qiong Xie's research group from the School of Pharmacy at Fudan University is the discovery of novel A2AR small molecule antagonists and their applications in cancer immunotherapy. In 2020 and 2022, they published a review (miniperspective) on A2AR antagonists for cancer immunotherapy (J. Med. Chem. 2020, 63, 12196−12212) and a research paper of novel pteridone A2AR antagonists (J. Med. Chem. 2022, 65, 4367−4386) in the Journal of Medicinal Chemistry, respectively.
Recently, Assoc. Prof. Qiong Xie, Prof. Yonghui Wang, and their collaborator, Prof. Weiqiang Lu from the School of Life Sciencesat East China Normal University,published a research paper in the Journal of Medicinal Chemistry, entitled “Discovery of Pyridinone Derivatives as Potent, Selective, and Orally Bioavailable Adenosine A2A Receptor Antagonists for Cancer Immunotherapy”. In this paper, a new class of pyridinone derivatives have been discovered as selective and orally available A2AR small molecule antagonists. Compared with the pteridone antagonists reported earlier by the team (J. Med. Chem. 2022, 65, 4367−4386), which had limited application due to low solubility, the pyridinone compounds reported in this paper not only had in vitro immunomodulatory effects on cells, but also exhibited good in vivo anti-tumor activities in animal models, and showed good oral absorption and excellent PK properties, which demonstrated therapeutic potential on cancer immunotherapy.
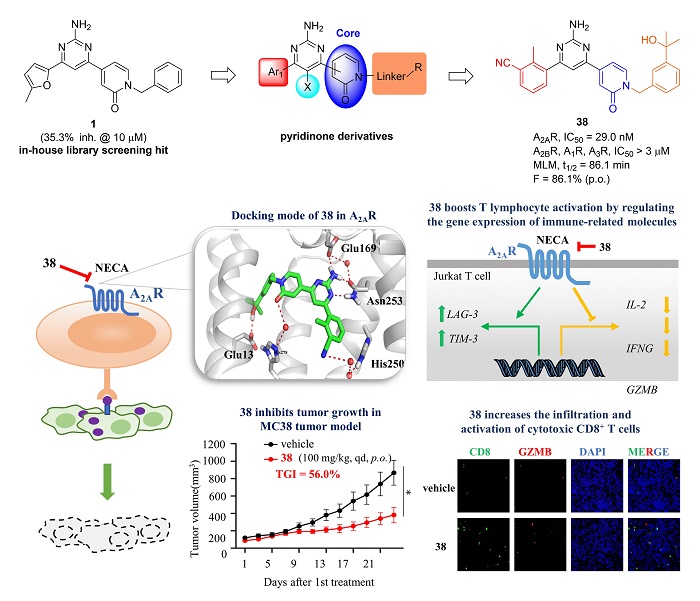
The discovery of pyridinone derivatives and their mechanism of action on cancer immunotherapy
In this study, by screening their in-house compound library, a pyridinone hit compound 1with weak A2AR antagonistic activity (μM level) was identified. Through further structure-activity relationship (SAR) studies and several rounds of structural modifications by classical medicinal chemistry strategies, compound 38, which had better activity and metabolic stability than the clinical compound CPI-444, was selected as the lead compound. Compound 38 stood out with a potent A2AR antagonistic activity (IC50 = 29.0 nM), good mouse liver microsomal metabolic stability (t1/2 = 86.1 min) and excellent oral bioavailability (F = 86.1%).
The docking study indicated that a large hydrogen bond network was engaged in the interactions between compound 38 and A2AR: the pyrimidine amino group forms key hydrogen bonds with Glu169 and Asn253, the cyano and carbonyl groups interact with His250 and His278 through water bridges, respectively, while the hydroxyl group forms a hydrogen bond with the carboxyl group of Glu13.
Further research on the mechanism of action indicated that compound 38 effectively enhanced the activation and killing ability of T cells in vitro by down-regulation of immunosuppressive molecules (LAG-3 and TIM-3) and up-regulation of effector molecules (GZMB, IFNG, and IL-2). Moreover, 38 exhibited excellent in vivo antitumor activity with a tumor growth inhibition (TGI) of 56.0% in MC38 tumor model via oral administration, and significantly increased the accumulation of CD8+T cells and the expression of granzyme B (GZMB) in tumors, demonstrating its potential as a novel A2AR antagonist candidate for cancer immunotherapy.
Chenyu Zhu (M.S. , School of Pharmacy, Fudan University), Ronghui Zhou (a master degree candidate from School of Pharmacy, Fudan University), and Shuyin Ze (a master degree candidate, School of Life Sciences, East China Normal University), shared the co-first authorship. Assoc Prof. Qiong Xie, Prof. Yonghui Wang (School of Pharmacy, Fudan University), and Prof. Weiqiang Lu (School of Life Sciences, ECNU) are the co-corresponding authors. This work was funded by the National Natural Science Foundation of China.
Article links: https://doi.org/10.1021/acs.jmedchem.2c01860
Introduction of Prof. Yonghui Wang and Assoc. Prof. Qiong Xie’s Group
The research group of Prof. Yonghui Wang and Assoc. Prof. Qiong Xie (School of Pharmacy, Fudan University) mainly focuses on the discovery of small molecule drugs for autoimmune diseases and tumor immunotherapy, and the research on new methods and technologies of organic and medicinal chemistry. Wang’s group is currently composed of 1 guest professor, 2 associate professors, 2 Ph. D. students and 4 master students. In recent years,7 students obtained their Ph. D. degrees and 5 students obtained their master’s degrees in this group. The research group has been funded more than 20 scientific research projects by the National Science and Technology Major Project, the National Natural Science Foundation of China, Shanghai Biopharmaceutical and Technology Supporting Plan, and the Natural Science Foundation of Shanghai, with a cumulative funding of more than 11 million yuan. In the past 5 years, Wang’s group has published more than 20 SCI papers in academic journals such as J Med Chem, Eur J Med Chem, J Org Chem, ACS Med Chem Lett, and applied for 16 Chinese patents (7 authorized) and 2 international PCT patent applications in the United States, Japan, and Europe (6 international patents have been authorized). In the research of small molecule drugs to treat autoimmune diseases and cancers, a number of drug candidates with potentials for further development have been discovered and are undergoing preclinical evaluation.
