
The recently emerged Omicron subvariants XBB and BQ.1.1 have presented striking immune evasion against most monoclonal neutralizing antibodies and convalescent plasma.Even three or four doses of parental messenger RNA (mRNA) vaccine cannot induce strong neutralization against XBB.The antibody evasion capability of some Omicron subvariants especially XBB has been demonstrated to reach or even exceed SARS-CoV. Therefore, the XBB has posed a significant challenge to the developed broad-spectrum COVID-19 vaccines.
Although almost 90% of neutralizing antibodies in COVID-19 convalescent sera are specific for receptor binding domain (RBD), lots of mutations of Omicron subvariants were observed on the RBD.
This has raised doubts about whether the RBD still contains highly conserved epitopes and, thus, whether it could be used as a proper immunogen to develop COVID-19 vaccines. Even if confirmed, how to stimulate these conserved epitopes to generate broadly neutralizing antibodies remains to be elucidated.

Recently, the research groups of Lu Lu, Shibo Jiang and Zezhong Liu from Fudan University published an article entitled“A pan-sarbecovirus vaccine based on RBD of SARS-CoV-2 original strain elicits potent neutralizing antibodies against XBB in non-human primates”in Proceedings of the National Academy of Sciences of the United States of America (PNAS). In this study, the authors have developeda pan-sarbecovirus vaccine that contains human Fc-conjugated RBD of ancestral SARS-CoV-2 strain as the immunogen and a novel STING agonist as the adjuvant (CF501/RBD-Fc). CF501/RBD-Fc could elicit extremely potent neutralizing antibodies against BA.2.2, BA.2.9, BA.2.12.1, BA.5, BA.2.75, and BF.7, as well as BQ.1.and XBB, which have both shown dramatic evasion of serum-neutralizing antibody activities. Meanwhile, the control vaccine, Alum/RBD-Fc, only elicited weak, or no, neutralization against BQ.1.1 and XBB. Notably, no significant difference (<4-fold) was observed in the neutralizing activity of sera in the CF501/RBD-Fc group against BA.2.2, BA.2.9, BA.5, BA.2.75 and BF.7 relative to D614G after 3 doses. Declines of 22.5-fold were found for XBB relative to D614G after 3 doses. The fold of reduction was significantly better than those reported before (66- to 155-fold) for the mRNA vaccines.
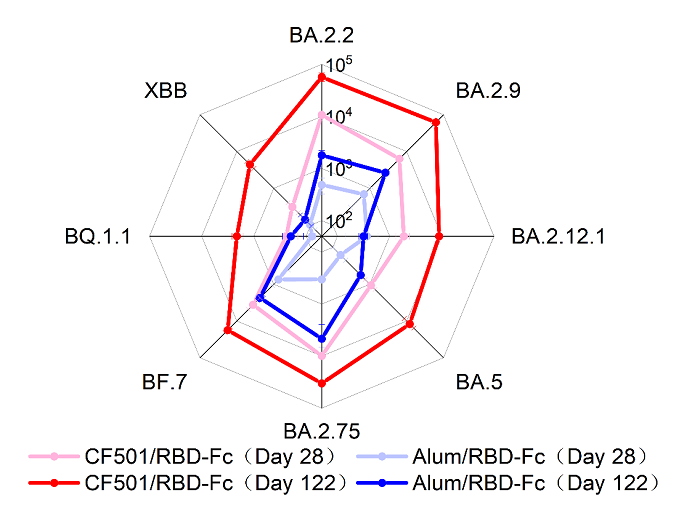
Fig 1. The radar figure showed the titer of nAbs elicited by CF501/RBD-Fc or Alum/RBD-Fc against Omicron subvariants at days 28 and 122 post the first vaccination.
In summary, the study supports that the “nonchangeable against changeable” strategy is feasible for the development of pan-sarbecovirus vaccines against sarbecoviruses, including the SARS-CoV-2 Omicron subvariants, BQ.1.1 and XBB. The study also implies that the adjuvant in the first-generation subunit COVID-19 vaccines might be replaced with CF501 and using it for boost immunization, which may significantly enhance the immune responses against SARS-CoV-2 and its current and future variants.
The first authors are Zezhong Liu, Jie Zhou and Xinling Wang from Fudan University.The corresponding authors are Zezhong Liu from the School of Pharmacy, Fudan University and Lu Lu, Shibo Jiang from the School of Basic Medical Sciences, Fudan University.
Link to original article https://doi.org/10.1073/pnas.2221713120