
Recently, the research group led by Prof. Chen Jiang has developed a bioinspired nanoerythrocyte, which could modulate the metabolic microenvironment of the brain lesion area and was applied for the whole process of acute ischemic stroke (AIS) treatment. The related article was published online in the internationally renowned journal Nano Today (IF=18.9) under the title of “Bioinspired nanoerythrocytes for metabolic microenvironment remodeling and long-term prognosis promoting of acute ischemic stroke”.
In recent years, the importance of the complex microenvironment of brain in the treatment of brain diseases has become increasingly prominent. Its basic unit is the neurovascular unit, which extends outward from the brain capillary endothelial cells that constitute the BBB, including pericytes, glial cells, nerve cells, and extracellular matrix. The mutual coupling and normal metabolic activities between cells are the basic elements to maintain the homeostasis of the microenvironment. Cell metabolites are important components of the microenvironment, including cytokines, gas molecules, and nutrients. The levels of these metabolites in turn influence the fate of the cells. Therefore, the metabolic homeostasis of cells in the microenvironment of the brain is critical to the overall microenvironment.
In AIS, the characteristics of the metabolic microenvironment mainly include the following two aspects. The first is abnormalities in neuronal metabolism, manifested by a marked downregulation of cellular oxygen, glucose and energy metabolism. This is followed by metabolic abnormalities in other cells in the microenvironment, such as glial cells and endothelial cells. Significant upregulation of reactive oxygen species, pro-inflammatory factors, and osmolarity was manifested. Metabolic abnormalities of other cells in the microenvironment will put metabolic stress on neurons, which will aggravate disease damage. Studies have shown that there is a unique internal mechanism of metabolic microenvironment damage in stroke. In the stage of hypoxia-ischemia, HIF-1α enters the nucleus in response to the hypoxic microenvironment, activates the expression of VEGF, and then causes the destruction of the BBB and the damage of microcirculation. In the later stage of reperfusion, with the excessive oxygen level, a large amount of ROS is produced, causing mitochondrial damage and ATP generation blockage. In addition, Akt/GSK-3β, a key signaling molecule in glucose metabolism, is dysregulated, leading to a decrease in glucose uptake and glycolysis. In summary, a basis is needed for the design of the drug delivery system by understanding the changes in the metabolic microenvironment of AIS, so as to treat the disease in a targeted manner.
Therefore, researchers designed the drug delivery system based on the following three key changes in the metabolic microenvironment (Figure 1). First of all, the BBB function is impaired in AIS, which is manifested by the presence of microthrombus deposition at the vascular injury, which suggests that thrombus-binding peptides can be utilized as the targeting head group of the drug delivery system to increase the residence time of the lesion in the brain. Secondly, the contradiction of oxygen metabolism in the metabolic microenvironment is mainly manifested in the double-edged sword role played by oxygen before and after reperfusion, and the hemoglobin in the organism is a natural oxygen regulator, and as a carrier of the drug delivery system, it can bind or releases oxygen reversibly. Furthermore, the level of glucose metabolism in neurons is significantly inhibited, and the model drug pyrroloquinoline quinone can activate the Akt/GSK-3β signaling pathway in cells, thereby correcting abnormal glucose metabolism in cells.
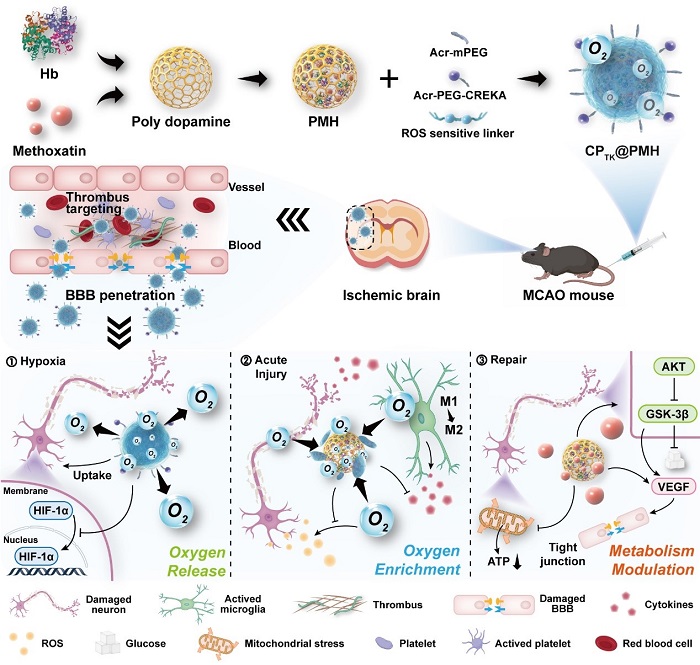
Figure 1. Schematic diagram of the construction of nanoerythrocytes and the modulation of the metabolic microenvironment in the whole process of AIS.
After targeting micro-thrombus, nanoerythrocytes could actively reach the ischemic core and enhance drug accumulation. During the ischemic phase, the nanoerythrocytes could release oxygen in response to the hypoxic microenvironment to relieve hypoxia. During the reperfusion phase, nanoerythrocytes could combine with excess oxygen to block the generation of ROS and promote the polarization of microglia, finally inhibiting the acute injury during the reperfusion phase. In the late recovery stage, nanoerythrocytes released drugs to regulate the Akt/GSK-3β signaling pathway to activate glucose metabolism and achieved BBB protection. During the progression of AIS, key metabolic elements, including oxygen balance and glucose metabolism, were positively regulated, and satisfactory short-term and long-term therapeutic effects were obtained in both pMCAO (permanent embolization model) and tMCAO (short-term embolization model) mouse models. Therefore, this work contributes to the understanding of the pathological process in different stages of AIS and suggests that metabolic microenvironment regulation can serve as a potential therapeutic strategy for AIS. More importantly, compared with recently developed nanomedicines, the concept of whole-course therapy can realize multi-target and multi-level treatment of diseases, which may have better clinical application potential.
Peixin Liu, PhD student of 2019 from School of Pharmacy, Fudan University, is the first author, while Prof. Chen Jiang is the corresponding author. The work was supported by thegrants from the National Natural Science Foundation of China (81872808, 82121002), Key Projects of Shanghai Science Foundation (19JC1410800), and Shanghai Municipal Science and Technology Major Project (Grant 2018SHZDZX01) and ZJLab.
Link address: https://www.sciencedirect.com/science/article/pii/S1748013223000555#ack0005