
Drug delivery systems (DDSs) are important pharmaceutical solutions for optimizing the in vivo drug distribution, where DDSs triggered by local microenvironment represents the state-of-art of nanomedicine design. Currently, the hot topics are mainly concerning the long circulation, fine tumor targeting and higher accumulation, as well as deep penetration. However, the design functioning post cellular entrance is still short of guiding theory and reviews. Recently, a review, entitled with “Drug delivery systems triggered by intracellular or subcellular microenvironment” was published on Adv Drug Deliv Rev authored by Prof Tao Sun and Prof Chen Jiang. In this review, drug delivery systems triggered by intracellular hallmarks were comprehensively reviewed, and the impressive progress was summarized, with the aim of providing reliable information for the clinical applications and structure design of the DDSs. The 1st author is Prof Tao Sun, and the corresponding author is Prof Chen Jiang.
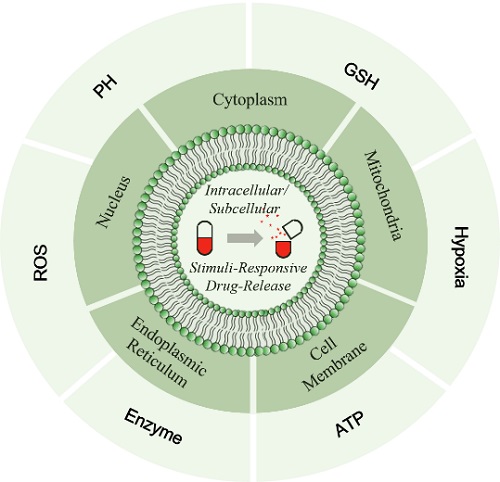
Scheme 1. Illustration on the stimuli-responsive drug delivery systems triggered by intracellular or subcellular microenvironments
An ideal DDSs could maintain the circulating stability, and achieve a burst release at the foci. Actually, the completely opposite demands promote the scientists to introduce so-called dynamic covalent bonds into DDSs, with a classic example: the pH drop and hypoxia enhancement in solid tumor could initiate the cleavage of the pH/hypoxia-responsive dynamic covalent bonds to trigger the drug release from DDSs at the aimed sites. In this regard, the cellular level is more microcosmic, and the intracellular sites are playing as most drug’s targets, which requires higher exquisiteness in the design by employing the triggering hallmarks at intracellular and subcellular levels.
This review starts from the current dilemma of nanomedicines’ translation, and raises the idea that intracellular drug fate should be paid with more attention at a new demission. The first half parts summarize the triggering hallmarks at intracellular and subcellular levels, including intracellular pH, glutathione (GSH), reactive oxygen species (ROS), hypoxia, enzymes, adenosine triphosphate (ATP), and so on; then, by listing recent reports, the stimuli-responsive DDSs at nucleus, mitochondria, endoplasmic reticulum and cell membrane are reviewed. Finally, the authors propose 7 aspects to this area, and point out the potential challenges and chances. This review includes nearly 200 high references. Hopefully, this review could give useful hints in developing nanoplatforms proceeding at a cellular level.
The authors believe the stimuli-responsive DDSs triggered by intracellular or subcellular microenvironments, could improve the drug fate control to a new dimension somehow, and provide useful hints to solve the problems on clinical translation and commercial availability.
This review has been accepted by Adv Drug Deliv Rev, which can be found via the link:https://www.sciencedirect.com/science/article/pii/S0169409X23000881?via%3Dihub