
Autophagy is a process of cellular scavenging that relies on lysosomes, whereby protein aggregates and damaged organelles are broken down. This process is evolutionarily conserved and serves to maintain cellular homeostasis, as well as promote cell survival in the face of stressors. Through the degradation of damaged organelles, misfolded or toxic proteins, and pathogens, autophagy plays a crucial role in the preservation of cellular health. As one of the most exciting and rapidly expanding fields of research in the biological sciences since the turn of the 21st century, autophagy has been linked to the pathogenesis of a number of human diseases, including neurodegenerative disorders and cancer.
The research group led by Prof. Renxiao Wang at our school is dedicated to the discovery and development of small-molecule regulators of protein-protein interactions with innovative opportunities in drug discovery. Over the past few years, the group has focused on the protein-protein interaction system involving ATG5 and ATG16L1 as their target. This system is critical to the extension of autophagic vesicles and the maturity of autophagosomes. Using targeted molecular design, they have successfully developed active compounds that could regulate the biological function of this target system. Their recent progress has been published in the Journal of Medicinal Chemistry, an esteemed publication in the field of medicinal chemistry produced by the American Chemical Society. The article, entitled “Discovery of small-molecule autophagy inhibitors by disrupting the protein-protein interactions involving ATG5” (https://doi.org/10.1021/acs.jmedchem.2c01233), highlights the team's groundbreaking progress in the development of small-molecule autophagy inhibitors.
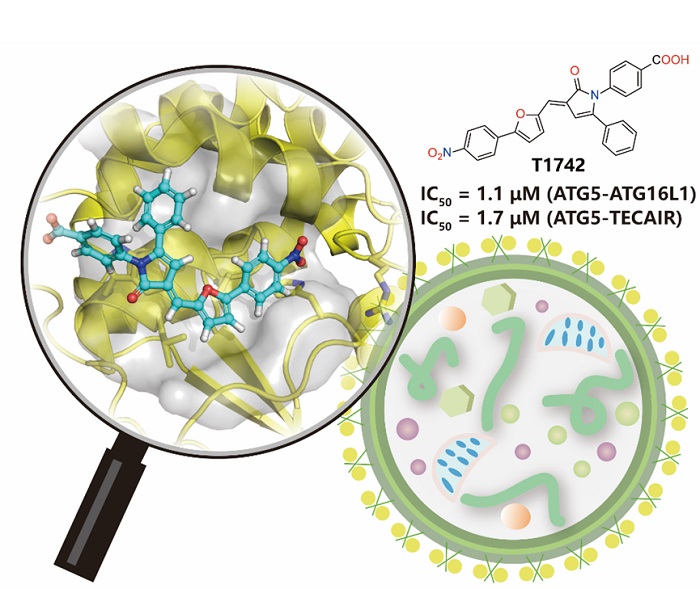
Using virtual screening, the authors identified a compound with an (E)-3-(2-furanylmethylene)-2-pyrrolidinone core moiety (T1742) that effectively blocked the interaction between ATG5-ATG16L1 and ATG5-TECAIR in binding assay (IC50 = 1~2 μ M). Further experiments using flow cytometry and western blot techniques confirmed that T1742 could inhibit autophagy in living cells in a dose-dependent manner. The authors also used molecular modeling to predict the possible binding mode of T1742 to ATG5 and accordingly synthesized a batch of derivatives sharing the same core moiety. The outcomes of the binding assay and the flow cytometry assay of the newly synthesized compounds were generally consistent, confirming the action mechanism of the active compounds. This work represents the first successful discovery of small-molecule inhibitors of ATG5 with bioactivities reaching the micromolar level. This class of ATG5 inhibitor may be used as a new tool to explore the mechanism of autophagy or have the potential to be developed into drug candidate.
This article has co-first authorship attributed to PhD candidates Honggang Xiang, Xiangying Zhang, and Ruiqi Liu. The co-corresponding authors are Prof. Renxiao Wang, Prof. Yingxia Li, and Prof. Lu Zhou. The research was conducted with assistance from Prof. Chun Guo's group at Shenyang Pharmaceutical University and was funded by both the National Natural Science Foundation of China and the Research Project of the Shanghai Science and Technology Commission.