
Liming Shao Group (School of Pharmacy, Fudan University) and Jinggen Liu Group (Shanghai Institute of Materia Medica, Chinese Academy of Sciences) has made progress in the study of novel κOR agonists and identification of compound SLL-1206 provides significant insights to the dissociation of analgesia from unexpected central nervous system (CNS) side-effects for κOR agonists, as well as a structural basis for development of potential analgesic candidates. This work has been published as a full-length article in Journal of Medicinal Chemistry (https://doi.org/10.1021/acs.jmedchem.1c01082).
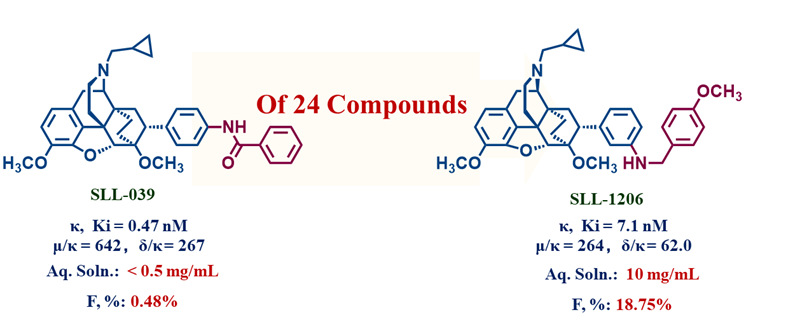
The search for selective kappa opioid receptor (κOR) agonists with reduced adverse CNS effects is one of the most intense areas of opioid research. Based on our previous efforts to identify a selective and potent kappa opioid receptor agonist (SLL-039) with a reduced sedation effect from N-cyclopropylmethyl-7α-phenyl-6,14-endoethano-tetrahydro northebaines and the Structure-Activity Relationship (SAR) analysis on structure-related compounds, a series of m-substituted analogs were designed, synthesized and assayed, resulting in the identification of compound SLL-1206 as a selective κOR agonist with single-digit nM activities. Compared to the p-substituted analogs, this series of m-substituted analogs displayed distinct structure-activity relationships (SARs) and receptor-binding modes. The subtype selectivity of this compound appeared to be a consequence of an enormous decrease in the affinity for μOR and δOR, rather than a significant increase in the affinity for κOR, which was not the case for SLL-039. However, the unique pharmacological characteristics of SLL-039, including functional activities in vitro and a κOR-mediated analgesia effect in vivo as well as reduced CNS side effects in rodent models could be reproduced by SLL-1206, suggesting that SLL-039 was not an outlier. This finding challenged our previous hypothesis that interaction with the subpocket at the top of transmembrane domain 2 (TM2) might play a role in the dissociation of analgesia and sedation among κOR agonists. More importantly, the physicochemical and pharmacokinetic properties of SLL-1206 were substantially improved compared to those of SLL-039, with increases of over 20-fold in aqueous solubility and approximately 40-fold in oral bioavailability in rats, which confirmed the potential utility of this compound in further studies.
Qian He, M.S. and Xiao Liu, Ph.D. (School of Pharmacy, Fudan University) and Yuanyuan Wei, Ph.D. (Shanghai Institute of Materia Medica, Chinese Academy of Sciences) shared the co-first authorship of this manuscript. This work was implemented under the supervision of Prof. Dr. Liming Shao and Assoc. Prof. Dr. Wei Li (School of Pharmacy, Fudan University), as well as Prof. Dr. Jinggen Liu and Prof. Dr. Yujun Wang (Shanghai Institute of Materia Medica, Chinese Academy of Sciences), and was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the National Natural Foundation of China and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
To cite this work
Qian He, Yuanyuan Wei, Xiao Liu, Rongrong Ye, Linghui Kong, Zixiang Li, Shuang Jiang, Linqian Yu, Jingrui Chai, Qiong Xie, Wei Fu, Yujun Wang, Wei Li, Zhuibai Qiu, Jinggen Liu, Liming Shao. Discovery of an m‑substituted N‑cyclopropylmethyl-7α-phenyl-6,14-endoethanotetrahydro northebaine as a selective, potent, and orally active κ‑opioid receptor agonist with an improved central nervous system safety profile. J Med Chem, 2021, 64(16), 12414–12433.
Related Papers
1.Liu X, Ye R.R., Kong L.H., et al. An Exploration of the SAR Connection Between Morphinan and Arylacetamide-based κ Opioid Receptor (κOR) Agonists using the Strategy of Bridging. ACS Chem NeuroSci, 2021, 12(6), 1018-1030. DOI: 10.1021/acschemneuro.1c00034
2.Xiao L, Wang Y.J., Zhang M.M., et al. Discovery of a highly selective and potent kappa opioid receptor agonist from N-cyclopropylmethyl-7α-phenyl- 6,14-endoethano-tetrahydronorthebaines with reduced central nervous system (CNS) side effects navigated by the message-address concept. J Med Chem,2019, 62(24): 11054-11070. DOI: 10.1021/acs.jmedchem.9b00857
3.Sun HJ, Wang YH, Yuan CM et al. 7β-methyl substituent is a structural locus associated with activity cliff for nepenthone analogues. Bioorg Med Chem, 2018, 26 (14), 4254-4263. DOI: 10.1016/j.bmc.2018.07.020
4.Li W, Long JD, Qian YY, et al. The pharmacological heterogeneity of nepenthone analogs in conferring highly selective and potent κ-opioid agonistic activities. ACS Chem NeuroSci, 2017, 8 (4), 766–776. DOI: 10.1021/acschemneuro.6b00321