
Plant derived natural products have continued to play a significant role in the drug discovery and development process. The opportunity for identifying new chemical entities (NCEs) for emerging diseases depends on a diversity of drug-producing species. Plant diversity loss significantly exacerbated the complications in the discovery of new natural products-derived drugs. Recently, several statistical surveys unveiled that the rare and endangered plants (REPs) have proven to be better sources for drug discovery than other botanic sources. An important goal for the conservation of the endangered plants is to provide key resources for new chemistry with utility in the control of new and emerging drug targets. Therefore, there is a tremendous need to prioritize protection and utilization of these fragile plant species.
Recently, Jin-Feng Hu’s group reported a novel class of [4+2] type pentaterpenoids derived from a rearranged lanostane moiety (dienophile) and an abietane unit (diene), namely forrestiacids A (1) and B (2). These unprecedented molecules were isolated using guidance by molecular ion networking (MoIN) from Pseudotsuga forrestii, an endangered member of the Asian Douglas-fir Family. The intermolecular hetero-Diels-Alder adducts feature an unusual bicyclo[2.2.2]octene ring system. Their structures were elucidated by spectroscopic analysis, GIAO NMR calculations with DP4+ probability analyses, electronic circular dichroism (ECD) calculations, and X-ray diffraction analysis. Forrestiacids represent one of the largest terpenoid molecules published to date, and expand the utility of computational chemical shift NMR approaches as effective and powerful strategies for assign and verify the correct NMR structure.
The elevated level of low-density lipoprotein cholesterol (LDL-C) represents a key risk factor for atherosclerotic cardiovascular disease (CVD), which is associated with high morbidity and mortality as well as increasing health-care costs. With the bempedoic acid (Nexlizet®) as the first non-statin ATP-citrate lyase (ACL) inhibitor approved by FDA, which is now prescribed for use in hypercholesterolemia, the ACL has emerged as a bright drug target for hyperlipidemia, hypercholesterolemia and other metabolic disorders.
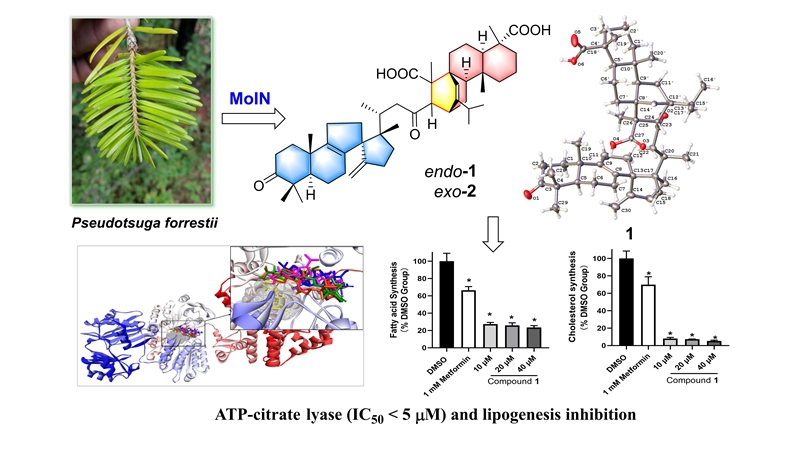
The two terpenoid [4+2]-adducts, forrestiacids A and B, displayed significant inhibition against ACL, with IC50 values of 4.12 and 3.57 μM, respectively. Interestingly, forrestiacids and bempedoic acid all are dicarboxylic acid derivatives. The major component, forrestiacid A (yield 0.008%), was further found to elicit dual inhibition on [14C]-labeled acetate incorporation into fatty acid and cholesterol in HepG2 cells, in a concentration-dependent manner. Remarkably, the treatment of forrestiacid A resulted in dramatic reductions in fatty acid (~75%, P < 0.05), and cholesterol (~93%, P < 0.05) syntheses at all test concentrations (10, 20, and 40 μM). These results provide a new chemical class for developing potential therapeutic agents for ACL-related diseases with strong links to traditional medicines.
A related molecular docking study was conducted to gain a deeper understanding of the mechanism of action (MOA). The result showed that, an allosteric site may be present in ACL as forrestiacids bind at the outer pocket of the citrate-binding site by interacting with different amino acid residues, whereas BMS 303141 binds at the citrate binding domain. While these [4+2]-adducts are clearly too large to enter into the citrate binding domain, they provide a unique site for new inhibitors of this important drug target.
These findings reveal the importance of protecting endangered plants as potential sources of new therapeutics. The relevant studies, entitled “Forrestiacids A and B, Pentaterpene Inhibitors of ACL and Lipogenesis: Extending the Limits of Computational NMR Methods in the Structure Assignment of Complex Natural Products”, were published in Angewandte Chemie International Edition, one of the prime chemistry journals in the world.
Dr. Juan Xiong, an Associate Professor from School of Pharmacy, Fudan University, is the first author. Professor Jin-Feng Hu (School of Pharmacy of Fudan University & School of Advance Study of Taizhou University), Professor Jia Li (Shanghai Institute of Materia Medica, Chinese Academy of Science), and Professor Mark T. Hamann (Medical University of South Carolina, USA) are the co-corresponding authors. This work was supported by the grants from National Natural Science Foundation of China (Nos. 21937002, 81773599, 21772025).
Link address: https: doi.org/10.1002/anie.202109082