
Lu Wang’s group in the School of Pharmacy, Fudan University, and Kai Johnsson’s Group in Max Planck Institute for Medical Research have developed a new strategy for synthesizing fluorescent probes for super-resolution microscopy. The new strategy can transform the same rhodamine into either a highly fluorogenic and cell-permeable probe for live-cell stimulated emission depletion (STED) microscopy or a spontaneously blinking dye for single-molecule localization microscopy (SMLM). The study was published in the Journal of the American Chemical Society (https://pubs.acs.org/doi/10.1021/jacs.1c05004).
Fluorescence microscopy is a commonly employed tool in medical and biological science. However, the spatial resolution of regular fluorescence microscopy is limited to about 200 nm due to the diffraction of light. Super-resolution microscopy, such as stimulated emission depletion (STED) microscopy and single-molecule localization microscopy (SMLM), breaks the diffraction limit of light and produces a nanometer spatial resolution, thus offering a powerful tool to investigate cellular structures, track single proteins, and analyze protein-protein interaction networks on a molecular scale.
Rhodamines are the most important class of fluorophores for applications in live-cell fluorescence microscopy. They exist in a dynamic equilibrium between a fluorescent “open” and a nonfluorescent but cell-permeable “close” form. In previous work (Nat Chem,2020,12,165), Dr. Lu Wang and Prof. Kai Johnsson reported a general strategy to transform regular fluorophores to cell-permeable and fluorogenic dyes. Conversion of a carboxyl group found in rhodamines and related fluorophores into an electron-deficient amide produces the close form, which can quickly penetrate cell membranes and show ultralow intracellular background signal. However, binding to target proteins shifts the equilibrium to the open state, thus producing several hundred-fold enhanced fluorescence. These probes have been commercialized by SpriochromeTMfor labeling protein tags, microtubules, and actins in wash-free, multicolor, live-cell nanoscopy.
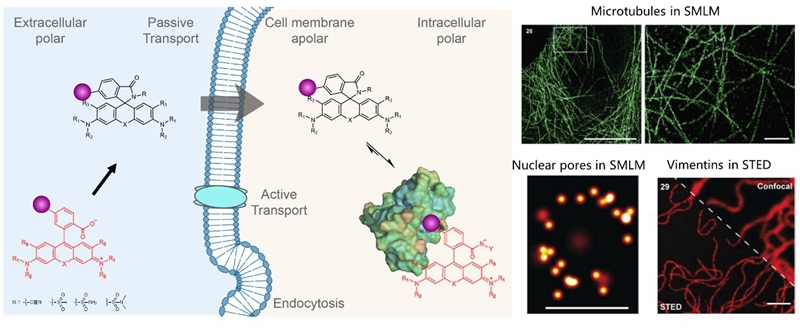 Figure 1. New fluorescent probes for wash-free, multicolor, live-cell super-resolution microscopy
Figure 1. New fluorescent probes for wash-free, multicolor, live-cell super-resolution microscopy
Recently, Lu Wang’s group and Kai Johnsson’s group extended the above strategy to systematically tune the equilibrium of open/close forms with unprecedented accuracy and over a large range.Incorporation of benzenesulfonamides with different substituents enabled the systematic fine-tuning of the open/close equilibrium and the fluorogenicity of the resulting probes for STED. Furthermore, the incorporation of more electron-rich amides allowed the tuning of the equilibrium over a large range and the generation of spontaneously blinking dyes for SMLM.
Such novel probes and super-resolution microscopy will provide a powerful tool to track drug target proteins in living cells and offer a novel drug screening platform. Prof. Kai Johnsson and Dr. Lu Wang are the co-corresponding authors. Nicolas Lardon is the first author.
About this article:
Nicolas Lardon, Lu Wang*, Aline Tschanz, Philipp Hoess, Mai Tran, Elisa D’Este, Jonas Ries, and Kai Johnsson*, Tuning of Rhodamine Spirocyclization for Super-resolution Microscopy.J. Am. Chem. Soc. 2021, doi.org/10.1021/jacs.1c05004.
https://pubs.acs.org/doi/10.1021/jacs.1c05004