
Glioblastoma (GBM) is the most common primary malignant glioma. As a molecular marker, isocitrate dehydrogenase 1 (IDH1) plays a key role in predicting the prognosis of GBM. The vast majority (>90%) of GBM patients belong to the IDH1 wild type (IDH1WT) group, and their median survival (about 13 months) is significantly lower than that of IDH1 mutated (IDH1MUT) patients (33 months). Although immunotherapeutic approaches have been proven to be effective in the treatment of multiple types of cancers, such as lymphoma and melanoma, these strategies, including immune checkpoint blockade and therapeutic vaccination, failed to achieve satisfactory therapeutic efficacy in glioma. The compromised therapeutic responses may result from the immunosuppressive microenvironment of GBM. Therefore, modulating the immunosuppressive microenvironment of IDH1WT GBM is a promising strategy in improving the immunotherapeutic response.
On June 6st, 2021, Cong Li’ s group reported their work “Reprogramming the immunosuppressive microenvironment of IDH1 wild-type glioblastoma by blocking Wnt signalling between microglia and cancer cells” in the journal of Oncoimmunology (https://www.tandfonline.com/doi/full/10.1080/2162402X.2021.1932061). This study demonstrated that compared with IDH1MUT GBM, the microglia with anti-inflammatory phenotype and the key gene CTNNB1 in the Wnt/β-catenin pathway were both highly expressed in IDH1WT GBM. To interrogate the potential relationship between anti-inflammatory microglia and β-catenin, they treated the microglia with IDH1WT GL261 GBM-derived conditioned media or IDH1MUT GL261 GBM-derived conditioned media, and found that IDH1WT glioma cells facilitate the transformation of primary microglia to an anti-inflammatory phenotype by activating the Wnt pathway. Then, Wnt-C59, an inhibitor of Wnt/β-catenin signaling, were added to the IDH1WT GBM cells and the anti-inflammatory microglia, respectively. Blockage of Wnt signaling not only triggered the phenotypic transition of immunosuppressive microglia into an immunostimulatory phenotype but also markedly mitigated IDH1WT GBM cell growth. Finally, gavage administration of Wnt-C59 enhanced cytotoxic CD8+ T cell infiltration, reduced Treg cell numbers, restrained glioma development and increased the survival of model mice bearing IDH1WT GBM allografts (Figure 1).
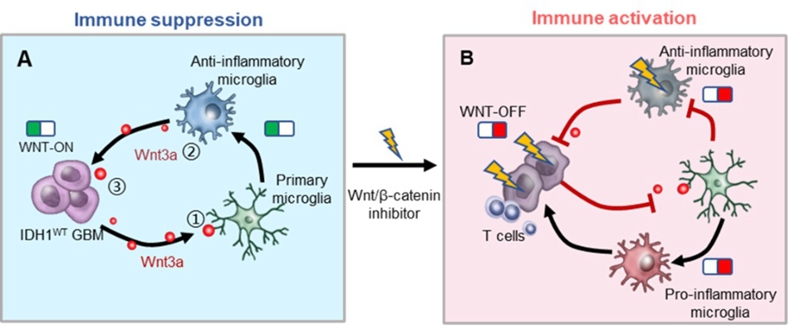
Figure 1. Wnt/β-catenin signaling blockade activates the immunosuppressive microenvironment of IDH1WT GBM.
This work indicates that Wnt/β-catenin signaling pathway plays an important role in maintaining the immunosuppressive glioma microenvironment. Blocking Wnt/β-catenin signaling is a promising complement for IDH1WT GBM treatment by improving the hostile immunosuppressive microenvironment.
Professors Cong Li (School of Pharmacy, Fudan University), Liang Chen (Department of Neurosurgery, Huashan Hospital, Fudan University) and Ruimin Huang (Shanghai Institute of Materia Medica, Chinese Academy of Sciences) are the co-corresponding authors of this article. Dandan Fan (a PhD candidate from the School of Pharmacy, Fudan University) and Dr. Yue Qi (Department of Neurosurgery, Huashan Hospital, Fudan University) are co-first authors.