
Epilepsy affects over 50 million people worldwide. Despite the availability of more than 20 antiepileptic drugs, approximately 30–40% of patients continue to suffer from recurrent seizures due to drug resistance. These individuals with drug-resistant epilepsy (DRE) are at heightened risk of comorbid neurological conditions, reduced quality of life, social stigma, and increased mortality. For patients with focal DRE, surgical resection of the epileptic foci (EF) remains one of the most effective therapeutic options. However, only 10–50% of DRE patients are suitable candidates for resection, and due to challenges in precise intraoperative localization and the high cost of surgery, fewer than 3% ultimately benefit from surgical treatment each year. Furthermore, incomplete resection of the EF results in persistent seizures in 30–50% of operated patients, underscoring the critical need for accurate intraoperative identification of epileptic tissues.
Noninvasive preoperative imaging modalities, such as magnetic resonance imaging and positron emission tomography, are commonly used to delineate EF but offer limited spatiotemporal resolution during surgery. Intraoperatively, factors such as brain shift can lead to inconsistent alignment between preoperative and intraoperative localization information, further making complete EF resection difficult. Electrocorticography (ECoG) remains the clinical gold standard for intraoperative localization, but its efficacy is hindered by several limitations:(1) the epileptiform discharges recorded during surgery are highly vulnerable to anaesthetic agents, which compromises the reliability of the collected EEG data; (2) prolonged sampling time is often required in order to obtain reliable result and may increase surgical risk, complications and surgeon fatigue; (3) the interpretation of ECoG results can be subjective, leading to empirical surgical resection. Therefore, an intraoperative, real-time strategy that can accurately predict the EF and its boundary could revolutionize epilepsy surgery by greatly improving the efficacy of entire EF resection.
In a recent study published in Cell Reports Medicine (titled “Ultrabright Ratiometric Raman-guided Epilepsy Surgery by Intraoperatively Visualizing Proinflammatory Microglia”), Cong Li, Cong Wang, and colleagues from Fudan University reported a novel intraoperative Raman imaging strategy for the precise visualization of EF. By analyzing clinical brain tissue from epilepsy patients, the researchers identified a marked accumulation of proinflammatory microglia and their key metabolic enzyme, myeloperoxidase (MPO), within EF. Leveraging computational screening, they developed an ultrabright, ratiometric surface-enhanced Raman scattering (SERS) nanoprobe capable of crossing the blood-brain barrier and specifically reporting MPO activity. This probe enabled quantitative imaging of proinflammatory microglia in situ, allowing for high-sensitivity and high-specificity localization of EF. In ex vivo patient brain tissue, the approach achieved a specificity of 93.3% and a sensitivity of 94.9%. Compared to clinical ECoG, this Raman-based strategy offers multiple advantages: (1) it is insensitive to anesthetic interference; (2) it provides a prolonged and stable imaging window exceeding one hour; (3) it directly visualizes the spatial distribution of MPO activity, translating molecular signatures into objective and intuitive Raman signals; (4) it can be readily integrated with other intraoperative modalities, such as neurophysiological monitoring, facilitating maximal resection of epileptogenic tissue while preserving eloquent cortex.
This work addresses a long-standing clinical challenge—the intraoperative identification of EF in DRE—by proposing a new paradigm centered on inflammatory microglial activity and its associated metabolic markers. The development of a ratiometric SERS nanoprobe enables in vivo, high-resolution, and quantitative visualization of epilepsy-associated molecular events, offering a powerful alternative to conventional electrophysiological approaches. The strategy demonstrates robust performance in both rodent models and freshly resected human brain tissue, presenting a compelling case for future clinical translation.
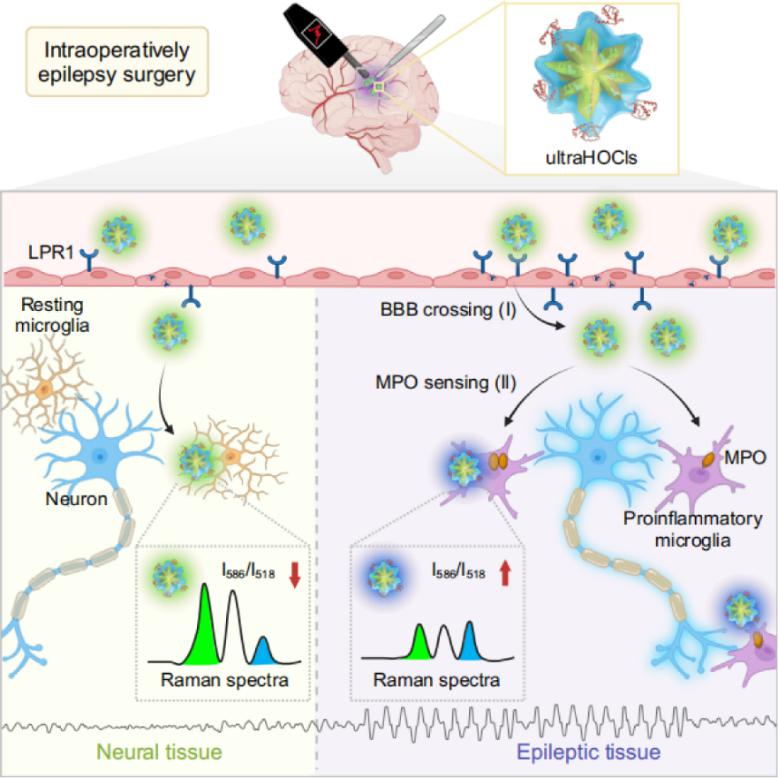
Figure 1 | Intraoperative localization of EF guided by ultrabright ratiometric SERS probes targeting proinflammatory microglia.
Dr. Cong Wang (School of Pharmaceutical Sciences, Fudan University) and Zhi Li (doctoral candidate, Sun Yat-sen University Cancer Center) contributed equally to this work. Prof. Cong Li and Dr. Cong Wang (School of Pharmaceutical Sciences, Fudan University); Prof. Ying Mao and Prof. Liang Chen (Huashan Hospital, Fudan University); Prof. Xiao Xiao (Institute of Brain-Inspired Intelligence, Fudan University); and Prof. Hairong Zheng (SIAT, CAS) are the co-corresponding authors of this article. This research was supported by the National Key Research and Development Program of China, Shanghai Municipal Science and Technology Commission, the National Science Fund for Distinguished Young Scholars, the National Natural Science Foundation of China, and other provincial-level funding.
Original article link: https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00228-9