
Understanding the spatiotemporal distribution of drug target proteins within cells is crucial for drug development and precision medicine. It enables rational drug design, optimizes therapeutic strategies, and minimizes off-target effects. However, due to the complex subcellular microenvironment, the localization and dynamics of target proteins can vary with the cell cycle, metabolic state, or external stimuli. Traditional population-level analyses and conventional microscopy (limited to ~200 nm resolution) fail to capture these dynamic functional states with sufficient precision.
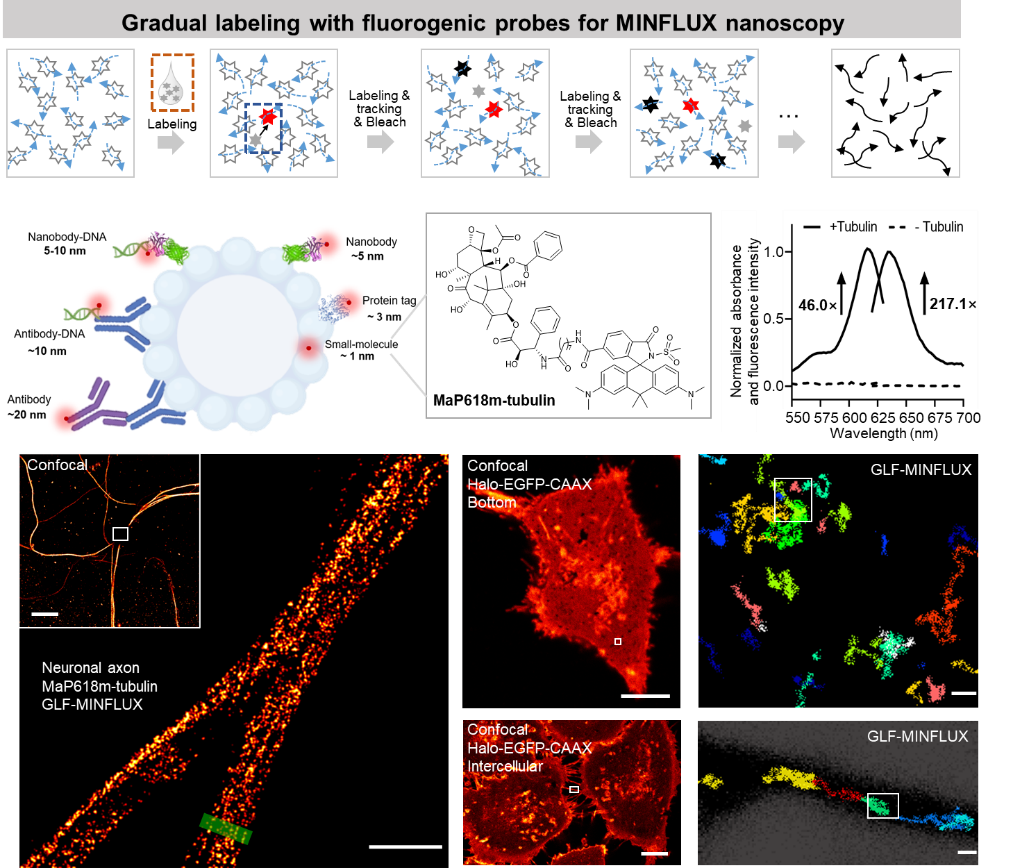
Figure 1. The mechanism of GLF-MINFLUX and its application in nanoscale imaging of drug targets
Recently, the research team led by Dr. Lu Wang at the School of Pharmaceutical Sciences, Fudan University, in collaboration with Professor Min Gu at the University of Shanghai for Science and Technology and Dr. Jiong Ma at Fudan University's Department of Optical Science and Engineering, published their latest research in Science Advances. They reported the development of a new super-resolution imaging technique—GLF-MINFLUX (Gradual Labeling with Fluorogenic Probes for MINFLUX)—which overcomes the dual bottlenecks of imaging resolution and labeling density in complex cellular environments, enabling high-precision visualization of drug target proteins.
This method integrates MaP fluorogenic dyes derived from the chemotherapeutic drug paclitaxel with protein-activated fluorescence switching and a “label–locate–bleach” workflow to achieve dense and precise labeling of microtubule proteins and other drug targets in living cells. Compared to traditional MINFLUX, GLF-MINFLUX improves localization precision by 1.7-fold, imaging efficiency by 2.2-fold, and labeling density by 3-fold, achieving spatial resolution as fine as 2.6 nm for targets such as microtubules and mitochondrial TOM20.
Moreover, GLF-MINFLUX enables dynamic tracking of membrane proteins in living cells with ~200 μs temporal resolution and ~7.8 nm spatial resolution. The study revealed distinct diffusion behaviors of membrane proteins, including "restricted" and "hopping" modes at the basal membrane and higher fluidity in filopodia, offering new insights into regional dynamics and transmembrane mechanisms of drug targets.
GLF-MINFLUX provides a powerful tool for real-time localization and dynamic analysis of drug targets in living cells, with broad potential applications in drug mechanism studies, signal transduction research, and the development of targeted therapies.
The study was co-first-authored by Dr. Longfang Yao, postdoctoral fellow at the University of Shanghai for Science and Technology, and Ms. Dongjuan Si, Ph.D. candidate at the School of Pharmaceutical Sciences, Fudan University. Dr. Lu Wang, and Dr. Jiong Ma from Fudan University and Professor Min Gu from University of Shanghai for Science and Technology, served as co-corresponding authors. The research was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Shanghai Basic Research Special Zone Program.
Read the full article: https://www.science.org/doi/10.1126/sciadv.adv5971