
Histone acetylation is one of the epigenetic regulation within cells, which is important for gene transcription, DNA replication, and DNA damage repair. This chemical modification is dynamically modulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs). E1A associated protein p300 (p300, EP300, KAT3B) and cyclic adenosine monophosphate response element binding protein binding protein (CBP, CREBBP, KAT3A) are closely related paralogous histone acetyltransferases and transcriptional co-activators. Through acetylation of H3K18 and H3K27 sites nearby the gene promotors and enhancers, and multiple transcriptional factors (TFs), p300/CBP activates gene transcription process. Abnormal transcriptional activation of enhancers upregulates oncogene expression, thereby altering a myriad of cellular events that promotes cancer development and progression, including cell cycle regulation and autophagy. Currently, cancer treatment strategy that target p300/CBP are focused on p300/CBP catalytic inhibitors and bromodomain inhibitors. Several p300/CBP bromodomain inhibitors are undergoing clinical investigations, with indications covering multiple hepatological malignancies and castration-resistant prostate cancer.
In recent years, Professor Li Yingxia’s Group at the School of Pharmacy, Fudan University, was dedicated to the discovery of p300/CBP inhibitors for cancer therapy, and have reported several series of p300/CBP inhibitors (Eur. J. Med. Chem. 2019, 180, 171–190, Bioorg. Chem. 2022, 124, 105803, Bioorg. Med. Chem. 2022, 66, 116784), accompanied by potency evaluation and mechanistic exploration. Recently, Professor Li’s group, in collaboration with Professor Huang Xun’s Group at Lingang Laboratory, reported a novel class of p300/CBP degraders with anti-hepatocellular carcinoma activity. This work was published in the Journal of Medicinal Chemistry entitled Discovery of Novel PROTAC Degraders of p300/CBP as Potential Therapeutics for Hepatocellular Carcinoma.
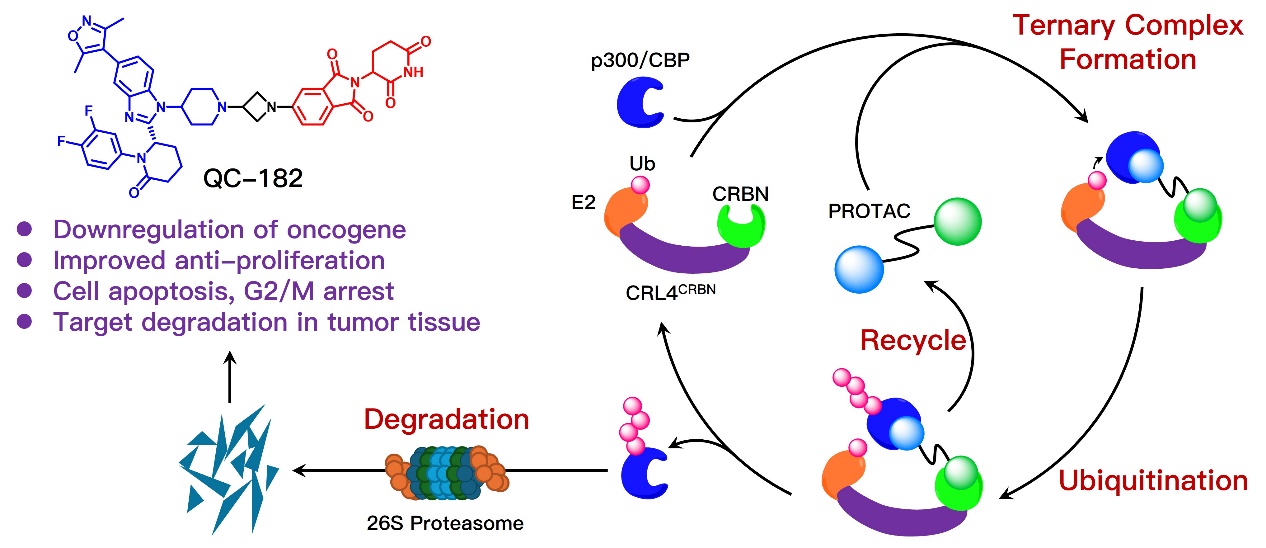
Accumulated studies have revealed additional domains involved in the oncogenic transcriptional activation within p300/CBP. Moreover, co-treatment of p300/CBP catalytic inhibitors and bromodomain inhibitors shows improved potency in cancer cells. Hence, protein degradation strategies, represented by proteolysis-targeting chimeras (PROTACs), are believed to elicit more profound anticancer efficacy. In addition, due to a lack of solid tumor investigation by targeting p300/CBP, the authors have screened sensitive cancer cell lines upon p300/CBP inhibition, and selected hepatocellular carcinoma (HCC) as target indication in this study.
This work employed the most advanced clinical p300/CBP bromodomain inhibitor CCS1477 as prototype binder for the design of p300/CBP PROTACs. Through the co-crystal structure analysis and biochemical assays, a suitable linking position was validated for the construction of PROTAC molecules. Degradation potency screening in HCC cells led to the identification of the promising p300/CBP PROTAC degrader QC-182, with effective activity on both p300 and CBP proteins via PROTAC mechanism. RNA-seq analysis revealed the treatment of QC-182 dramatically downregulates p300/CPB-associated transcriptome, essentially those involved in epithelial−mesenchymal transition and G2/M checkpoint in HCC cells. QC-182 shows superior antiproliferative potency compared to prototype CCS1477 and newly reported degrader dCBP-1, and could induce cell apoptosis and G2/M arrest. In vivo study demonstrated the effectiveness of p300 and CBP protein degradation by QC-182 in mouse tumor tissue. In summary, QC-182 is a potent p300/CBP PROTAC degraders, warranting further optimization toward the discovery of lead compound for HCC and other solid tumors.
Chang Qi, a PhD candidate at the Department of Medicinal Chemistry, School of Pharmacy, Fudan University, and Li Jiayi and Deng Yue, MS students at Shanghai Institute of Materia Medica, Chinese Academy of Sciences, are co-first authors of this article. Professor Li Yingxia and Professor Huang Xun are joint corresponding authors. This work was supported by grants from National Natural Science Foundation of China and Science and Technology Commission of Shanghai Municipality.
Original Article URL: https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c01468