
KRAS is one of the most prevalent oncogenic drivers of human malignancy including lung cancer. Approximately 25% of NSCLC harbor the mutated KRAS gene (KRASmut), among which KRASG12C is the most common type in lung adenocarcinoma (LUAD). Tissue factor (TF) activation can greatly promote the malignant behavior of cancer and plays an increasingly important role in the tumor microenvironment (TME) to influence the outcome of targeted therapies.
One of the research directions of Prof. Ker Yu's group at the School of Pharmacy, Fudan University, is the creation of new drugs with Tissue Factor TF-Therapeutic Antibodies and their role and mechanism in tumor therapy. The team recently published a research paper entitled "Tissue factor overexpression promotes resistance to KRASG12C inhibition in non-small cell lung cancer" in the journal Oncogene (original link: https://www.nature.com/articles/s41388-023-02924-y).The study reveals a role for the TF-mTORC2 axis in the suppression of TF protein degradation and negative regulation of macrophage phagocytosis, leading to impaired immune escape and tumor cell killing. This effect can be reversed by TF knockdown or drugs targeting the TF/mTOR axis to achieve a significant improvement in the anti-tumor efficacy of KRASG12C inhibitors, identifying a novel and important mechanism of resistance to KRASG12C inhibitors.

In this study, the researchers established a link between TF and drug-resistant tumors through transcriptome and tissue microarray analysis. They used a clinical stage TF antibody HuSC1-39 and the mTORC1/2 inhibitor MTI-31 (SCC31) to explore the functional role of TF in KRAS-mutant NSCLC, and found that HuSC1-39 had significant efficacy in overcoming KRAS inhibitor resistance in vitro and in vivo, and that remodeling of tumor microenvironment (TME) may be involved in this setting.

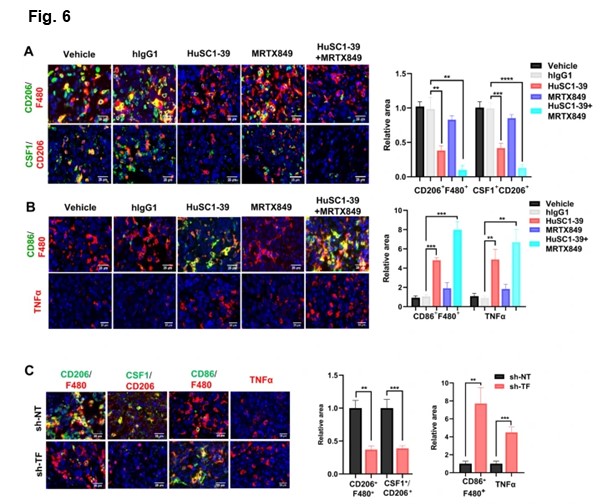
Tumor microenvironment is rich in tumor-associated macrophages (TAMs). Most TAMs are tumor-promoting M2-like macrophages, while tumor suppressor M1-like macrophages are the minority. CSF1 is the primary tumor-derived factor responsible for M2 recruitment. They observed that although the total level of macrophages (F4/80+) was not substantially affected, the number of M2-macrophages (F4/80+CD206+) and CSF1 were dramatically reduced and the activated M1-macrophages (F4/80+/CD86+) were increased in HuSC1-39 or MTI-31 treated tumors. This M1-promoting mechanism was also observed in the TF-depleted tumors.
As demonstrated by immunofluorescence, there were significantly increased amounts of TNF-α secretion in tumors treated with HuSC1-39, MTI-31 or TF-depletion. Combination therapy with KRAS G12C inhibitor MRTX849 resulted in a further reduction of intra-tumor M2-macrophages, exhibiting a stronger M1-macrophage phenotype with highest levels of TNF-α. Taken together, HuSC1-39 and MTI-31 exert their antitumor effects by altering the M2/M1 macrophage ratio and activating M1 macrophage function to overcome KRAS inhibitor resistance.These data further support the importance of the mTORC2-TF axis in disease progression and therapy resistance in KRASmut tumors.
Yu Zhang, a 2019 PhD student from the School of Pharmacy, Fudan University, is the first author of this paper, and Professor Ker Yu from the School of Pharmacy, Fudan University, is the corresponding author of this paper. This work was funded by NSFC, NST Major Project of ChinaandScience and Technology Achievement Transformation.