
Glioma is the most common malignant primary brain tumor, with surgical resection being the first-line treatment. However, due to the invasive and heterogeneous nature of glioma cells within the brain, accurately defining tumor margins remains a major clinical challenge. Incomplete resection may leave malignant tissue behind, leading to rapid recurrence, while excessive resection risks damaging neural function. The 2021 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (5th edition) emphasizes molecular markers in glioma classification, with the isocitrate dehydrogenase 1 (IDH1) gene serving as a core criterion. IDH1, a rate-limiting enzyme in the tricarboxylic acid (TCA) cycle, when mutated (e.g., R132H), produces an oncometabolite, D-2-hydroxyglutarate (D-2HG). This metabolite disrupts DNA and histone demethylation, leading to epigenetic dysregulation, dedifferentiation, and uncontrolled proliferation of tumor cells. Rapid intraoperative IDH1 genotyping is clinically valuable for improving surgical outcomes. IDH1 wild-type tumors generally respond poorly to postoperative chemoradiotherapy and require maximal safe resection. In contrast, IDH1-mutant tumors are more responsive to adjuvant therapies, and surgeons aim to preserve neural function while achieving precise removal. Therefore, rapid IDH1 genotyping during surgery is crucial for optimizing glioma treatment strategies.
Current gold standards for IDH1 genotyping—such as immunohistochemistry and gene sequencing—typically take 2–5 days to deliver results, limiting their utility for intraoperative guidance. Magnetic resonance spectroscopy imaging (MRSI) can detect D-2HG to infer IDH1 mutation status but suffers from high technical demands, long processing time, and susceptibility to interference. While desorption electrospray ionization mass spectrometry (DESI-MS) enables intraoperative D-2HG quantification, it cannot capture spatial heterogeneity within the tumor. Thus, there is a pressing need for a rapid, precise, and intraoperative IDH1 genotyping technology to support personalized surgical strategies.
The IDH1 mutation not only abolishes the enzyme’s normal catalytic function but also confers a neomorphic activity—reducing α-ketoglutarate (α-KG) to D-2HG. This metabolic rewiring leads to D-2HG accumulation and disrupts the cell’s redox balance. Compared to wild-type cells, IDH1-mutant glioma cells exhibit elevated reactive oxygen species (ROS) and decreased levels of the antioxidant glutathione (GSH), providing a novel metabolic signature for intraoperative IDH1 genotyping.
Recently, Prof. Cong Li’s team from the School of Pharmacy, Fudan University, in collaboration with Jinhua Yu, Xiao Zhu, and Cong Wang, published a study in Advanced Science titled: “Artificial Intelligent-Enhanced Metabolite Profiling for Intraoperative IDH1 Genotyping in Glioma Using an Orthogonally Responsive SERS Probe.” In this study, the team introduced an innovative strategy that simultaneously quantifies intracellular redox-related metabolites to rapidly identify IDH1 genotypes in glioma. They designed an orthogonally responsive surface-enhanced Raman scattering (SERS) probe capable of detecting extracellular levels of both GSH and hydrogen peroxide (H₂O₂). A custom deep neural network named DBCNet was developed to convert Raman spectra into 2D images for feature extraction, leveraging a multi-branch architecture and metric learning algorithm to enhance spectral feature clustering. Together, the SERS probe and DBCNet algorithm enabled simultaneous quantification of multiple tumor metabolites, achieving rapid IDH1 genotyping within just 7 minutes during surgery. In a clinical study involving 31 glioma patients, the method demonstrated an area under the receiver operating characteristic (ROC) curve of 0.985, offering a powerful tool to optimize surgical decisions and guide individualized treatment strategies.
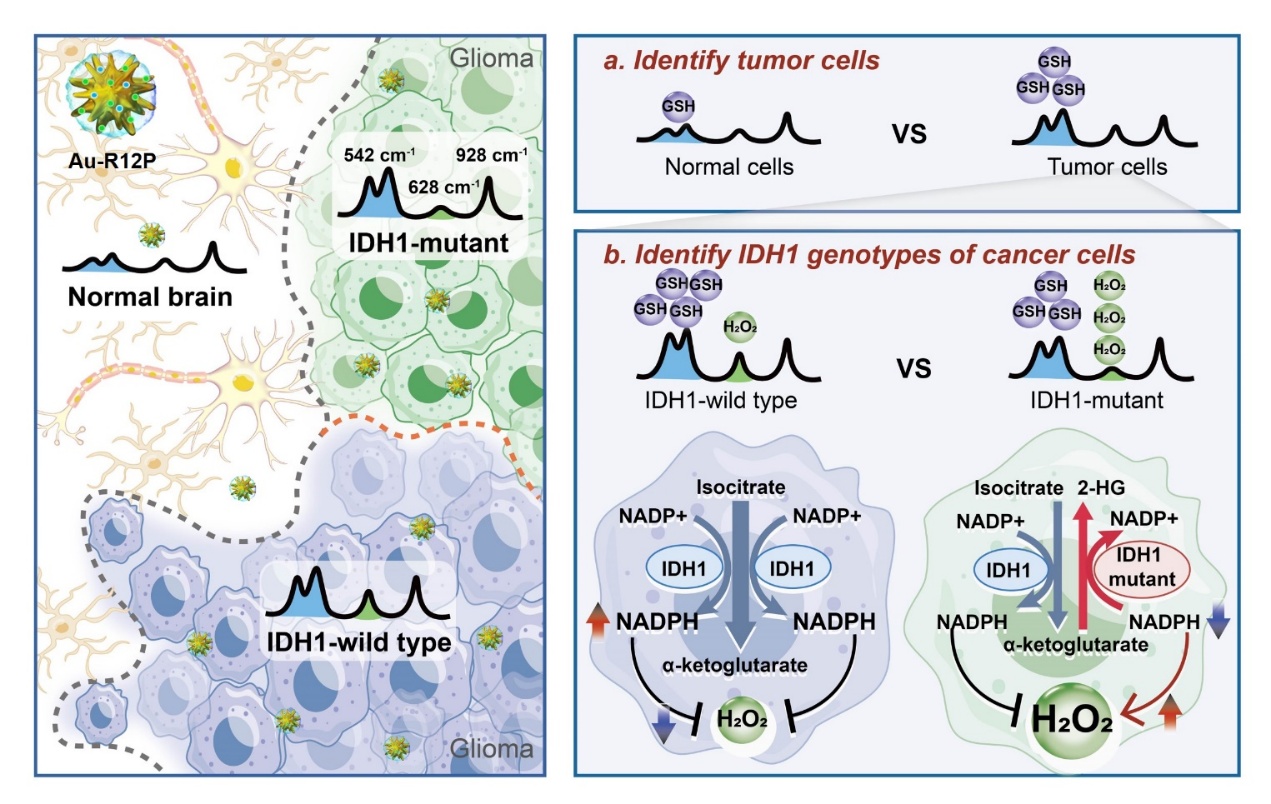
Figure 1. Schematic illustration of simultaneous detection of multiple tumor redox metabolites enables rapid intraoperative genotyping of IDH1 in glioma.
Hang Yin (Ph.D. student, School of Pharmacy, Fudan University), Dr. Xin Zhang (Huashan Hospital), Zheng Zhao (Master’s student, School of Information Science and Engineering, Fudan University), and Dr. Chong Cao (Shanghai Ninth People’s Hospital), are co-first authors of the paper. Prof. Cong Li, Dr. Xiao Zhu, and Dr. Cong Wang from the School of Pharmacy at Fudan University, Prof. Jinhua Yu from the School of Information Science and Engineering at Fudan University, and Prof. Ying Mao, chief neurosurgeon at Huashan Hospital, are co-corresponding authors. This work was supported by the National Key Research and Development Program of China, the National Science Fund for Distinguished Young Scholars, and Shanghai Science and Technology Projects.