
In the treatment of resectable solid tumors, surgery remains the basic and critical intervention. However, for highly invasive tumors, such as gliomas, which are located in the central nervous system and have indistinct boundaries, it is often difficult to completely remove the tiny residual lesions, leading to a high incidence of recurrence. Current local therapeutic strategies (e.g. immunotherapy, nanomedicines) show promise but face challenges, including the difficulty of precise drug residency and high systemic toxicity and side effects, which limit their efficacy.
Recently, Professor Rongqin Huang from School of Pharmacy at Fudan University, in collaboration with Professor Wei Tao from Harvard Medical School, has published a research article in the journal Cell Biomaterials. The paper titled "Modulating nano-bio interface via drug-framework hybridization to activate chemoimmunotherapy against glioma postoperative recurrence", explores the potential of drug-framework hybridization to enhance chemoimmunotherapy against glioma recurrence post-surgery. The study's central premise is the development of a novel drug-framework hybrid nanomaterial, termed DHSF, which integrates chemotherapy with immunotherapy to provide a safe and long-lasting localized treatment strategy for suppressing tumor recurrence after surgery. This work developed a drug-framework hybrid nanomaterial, DHSF, which provides a safe and long-lasting local therapeutic solution to inhibit postoperative recurrence of tumors by combining chemotherapy and immunotherapy. The DHSF material is a distinctive organic-inorganic hybrid, synthesized by integrating chemotherapeutic agents into mesoporous frameworks. This integration results in the formation of a stable backbone, facilitating the sustained and controlled release of the drugs within the tumor microenvironment. The hybridization approach ensures the degradation of the drugs occurs at a rate that is both low and prolonged, thereby significantly minimizing the likelihood of tumor recurrence. Additionally, the prolonged release profile contributes to a substantial reduction in toxicity, while concurrently and precisely inhibiting immune checkpoints (e.g., PD-L1). In addition, DHSF activates the TLR4-NFκB pathway through enhanced binding to the TLR4 receptor, stimulating immune cells to secrete chemokines and further enhancing the anti-tumor immune response. Notably, DHSF demonstrates exceptional tissue adhesion and hemostatic ability. Leveraging these characteristics, the research team further developed a photocurable hydrogel formulation capable of immediate intraoperative cavity filling, forming a "drug-immune depot" that provides sustained local chemotherapy and immune activation, thereby enabling precise intervention against postoperative recurrence. Animal studies demonstrated that this hydrogel significantly delayed tumor recurrence and prolonged survival in post-operative glioma models. It also demonstrated excellent biocompatibility and metabolic clearance with no detectable toxicity in major organs such as the heart, liver or kidneys. These results validate its broad clinical potential for intra-operative application, postoperative sustained release and post-operative immune modulation.
The DHSF hybridization strategy proposed in this study not only optimizes the bio-interfacial properties and drug release behaviors of traditional nanomedicines, but also provides a new design concept for postoperative local combination therapy. The potential of this strategy is expected to be extended to the postoperative management of other highly recurrent solid tumors, such as hepatocellular carcinoma, pancreatic carcinoma and ovarian carcinoma, which will provide a solid support for clinical translational research in the related fields.
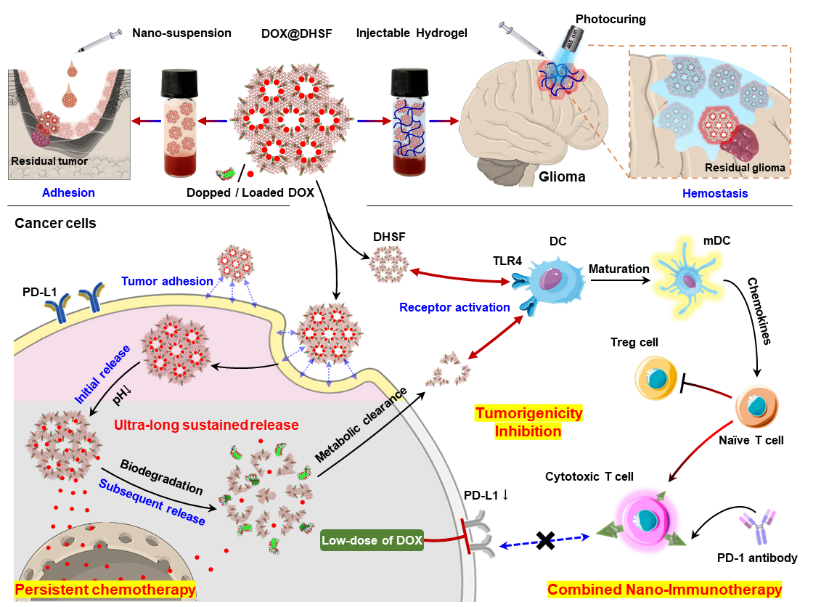
Scheme 1: Diagram of the drug-framework hybridization strategy. The design process and mechanism of DHSF are demonstrated, including the synergistic hybridization of small molecule drugs with mesoporous skeleton to form an organic-inorganic integrated structure, and can be designed as a locally injected nano-suspension or post-implantation light-cured gel to adapt to different therapeutic needs in the postoperative invasive cavity. The system can respond to degradation in the tumor microenvironment to achieve multiple therapeutic functions such as sustained drug release, immune activation and recurrence inhibition.
Min Qian (PhD candidate, School of Pharmacy, Fudan University), Guangwei Jiang (Master’s student, School of Pharmacy, Fudan University), and Senfeng Zhao (Harvard Medical School) contributed equally to this work. Professor Wei Tao (Harvard Medical School) and Professor Rongqin Huang (Fudan University) served as co-corresponding authors. The research was supported by grants from the National Natural Science Foundation of China and the Shanghai Municipal Science and Technology Major Project.
Link to paper: https://www.cell.com/cell-biomaterials/fulltext/S3050-5623(25)00016-9