
Epilepsy, one of the most common neurological disorders causing death and disability, is characterized by disrupted excitation–inhibition balance and aberrant synchronous neuronal discharges. Current antiepileptic therapies primarily target neuronal ion channels or neurotransmitter receptors, offering only symptomatic relief without halting disease progression or achieving long-term remission. Accumulating evidence highlights the pivotal role of extracellular pH homeostasis in modulating neuronal excitability, synaptic plasticity, glial function, and neurodegenerative processes. Under physiological conditions, brain extracellular pH is tightly regulated within 7.2–7.4; even subtle deviations of 0.2 pH units can markedly influence ion channel conductance and enzymatic activity, thereby impacting neuronal excitability. Notably, pH responses vary across neuronal subtypes: while an alkaline milieu can potentiate neuronal firing, acid-sensitive neurons exhibit increased activation under acidic stress. These findings underscore the importance of real-time monitoring of pH fluctuations in epileptic lesions, which may shed light on the intricate coupling between pH dynamics and neuronal excitability and offer mechanistic insights into epileptogenesis.
Although several technologies have been developed for in vivo brain pH sensing, they present significant limitations: invasive microelectrode implantation, limited temporal resolution of MRI-based methods, and the poor brain-penetration efficiency and quantification challenges associated with fluorescence imaging. Therefore, there is an urgent need for noninvasive imaging modalities capable of penetrating the blood–brain barrier (BBB) and sensitively detecting focal pH alterations in pathological brain regions.
Surface-enhanced Raman scattering (SERS), leveraging localized surface plasmon resonance on noble metal nanostructures, enables ultrasensitive molecular detection. Through surface functionalization, SERS probes can achieve enhanced biocompatibility, BBB permeability, and lesion specificity. Importantly, chronic neuroinflammation—a common pathological hallmark across multiple neurological disorders—is characterized by elevated hydrogen peroxide (H₂O₂) levels, making H₂O₂-responsive probes an attractive strategy for lesion-specific targeting and dynamic monitoring of the inflammatory microenvironment.
In a recent publication in Angewandte Chemie, Dr. Cong Wang and Prof. Cong Li from the School of Pharmacy at Fudan University, together with Prof. Zuhai Lei (Yunnan University) and Prof. Jing Wang (Institute of Brain-Inspired Intelligence), reported a study entitled “Tandem Dual-Ratiometric SERS Probe Enables Raman Imaging of Neurological pH Fluctuations in Epilepsy”. The team developed a tandem dual-ratiometric SERS probe (RHP@AuS) that enables real-time visualization of dynamic pH oscillations in epileptic brain regions and evaluation of therapeutic outcomes. Key innovations of this work include: (1) A self-assembly strategy based on dendritic Raman reporters, which effectively prevents signal quenching caused by molecular aggregation and significantly enhances Raman signal intensity and stability. (2) The design of a H₂O₂-gated, dual-ratiometric SERS probe capable of precise localization within epileptic foci and digital decoding of pH variations. (3) The discovery that epileptic seizures induce rapid local acidification in lesioned brain regions, leading to the proposal of a novel combined therapeutic strategy targeting both pH homeostasis and neuroinflammation, achieving sustained seizure suppression. Collectively, this work establishes a high-sensitivity, high-specificity Raman platform for accurate and dynamic monitoring of pH fluctuations in neurological disorders, opening new avenues for precision diagnosis and therapy of epilepsy and related brain diseases.
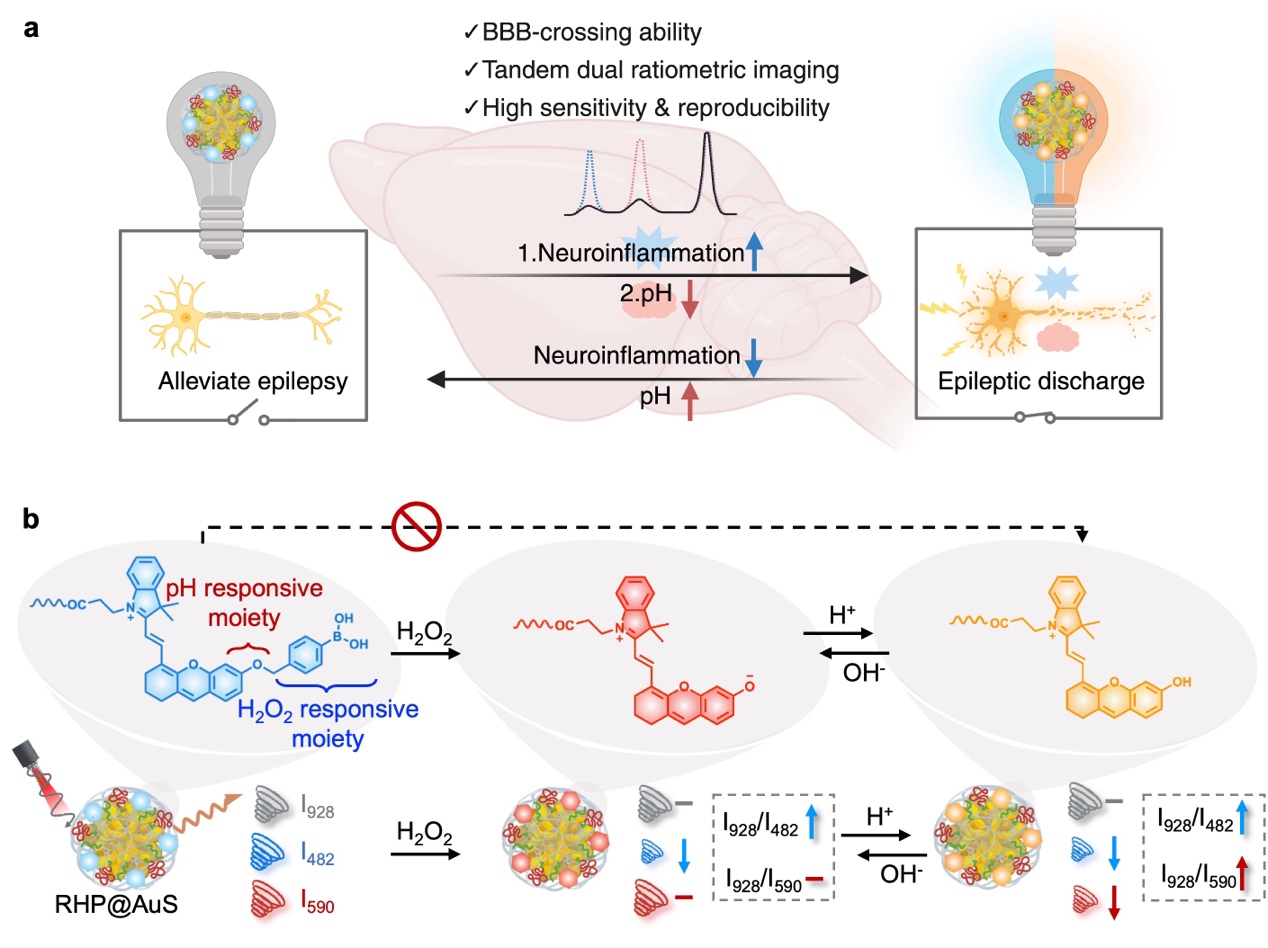
Figure 1. Tandem dual-ratiometric SERS probe enables dynamic monitoring of pH fluctuations in epileptic neurons.
Jing Zhao (Ph.D. student, School of Pharmacy, Fudan University), Yuncan Chen (Ph.D. student, Huashan Hospital), and Yurui Tang (Master’s student, Institute of Brain-Inspired Intelligence) contributed equally to this work. Cong Wang, Cong Li, Zuhai Lei, and Jing Wang are the corresponding authors. The study was supported by grants from the National Key R&D Program of China, the National Science Fund for Distinguished Young Scholars, the National Natural Science Foundation of China, and the Science and Technology Commission of Shanghai Municipality.
Original article link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202504822