胰高血糖素样肽-1受体结构与功能研究新进展
Latest progress in the study on the glucagon-like peptide-1 receptor
2018年 6月15日,中国科学院上海药物研究所/复旦大学药学院和澳大利亚莫纳什大学药学研究所两个团队关于胰高血糖素样肽-1受体偏向激活的合作研究成果以“Two distinct domains of the glucagon-like peptide-1 receptor control peptide-mediated biased agonism”为题发表在The Journal of Biological Chemistry上。
A collaborative study entitled “Two distinct domains of the glucagon-like peptide-1 receptor control peptide-mediated biased agonism” carried out by two research teams at Shanghai Institute of Materia Medica, Chinese Academy of Sciences/School of Pharmacy, Fudan University and Monash Institute of Pharmaceutical Sciences, Australia, was published by The Journal of Biological Chemistry on June 15, 2018.
G蛋白偶联受体是具有七次跨膜结构的膜蛋白家族,胰高血糖素样肽-1受体属于其中的B1类类型,为2型糖尿病和肥胖症的治疗靶点,已经上市的该受体激动类药物包括艾塞那肽、利拉鲁肽和度拉糖肽等近十种,年销售额超过百亿美元。这些药物所展现的不同给药频率、临床疗效、不良反应和耐受性与胰高血糖素样肽-1的偏向激动效应密切相关。
G protein-coupled receptors (GPCRs) belong to a family of membrane proteins characteristic of seven transmembrane domains. Glucagon-like peptide-1 receptor is a member of the glucagon receptor subfamily of class B GPCR and has been implicated in the treatment of type 2 diabetes and obesity. A handful of therapeutic agents, namely, glucagon-like peptide-1 receptor agonists, such as Exenatide, Liraglutide, Dulaglutide, etc. are presently on the market with annual sales of billions of dollars. These drugs require different administration frequency and possess variable efficacies, adverse event as well as tolerance, which may be related to the outcome of biased agonism of glucagon-like peptide-1 receptor.
偏向激动系指不同激动剂与受体结合使其构象发生不同的变化并偶联不同的效应蛋白,由此激活不同的信号通路或偏好激活某条信号通路,进而产生不同的生物效应。胰高血糖素样肽-1受体的N端和第一跨膜螺旋近胞外结构域于其激活过程中动态变化且极不稳定,在多个已经解析的晶体或冷冻电镜三维结构中都有缺失,对相关氨基酸位点的作用认识匮乏。
Biased agonism is a term describing the phenomenon that GPCRs are highly dynamic proteins and can adopt numerous ligand-specific conformational ensembles with distinct impact on signaling and regulatory profiles, even with ligands acting via a common binding pocket. The N-terminal and the residues on transmembrane loop 1 (TM1) near the extracellular domain of glucagon-like peptide-1 receptor are very flexible and usually missing in the three-dimensional crystal or Cryo-electron microscopy (cryo-EM) structures of the receptor resolved thus far. Their roles in the full-length wild-type receptor activation are not clear.
为了突破这个难题,王明伟课题组与澳大利亚莫纳什大学Patrick Sexton和Denise Wootten课题组三年前开始组织联合攻关,对该受体N端和第一跨膜螺旋近胞外结构域的28个氨基酸进行定点突变,采用多种技术系统研究了多种肽类配体对受体亲和力及不同信号通路(cAMP、pERK1/2和Ca2+)的激活效能。结果显示,胰高血糖素样肽-1受体上不同的氨基酸位点群调控着配体的亲和力及其所激活的信号通路,配体之间和信号通路之间既有共性也有特性,说明该结构域对信号偏向转导起着重要的调节作用,从而为基于受体偏向激动原理设计趋利避害的药物提供了新的思路。
To overcome this hurdle, scientists let by Drs. Ming-Wei Wang, Patrick Sexton and Denise Wootten started the joint effort three years ago. Focusing on the N-terminal and TM1 region near the extracellular domain, they mutated 28 residues and used several techniques to systematically study receptor binding affinities and activation efficacies on different signaling pathways (cAMP, pERK1/2 and Ca2+) of several peptidic ligands. It was found that different residue groups were able to modulate both affinity and signaling in a varied but overlapping manner, and different ligands bind to the same receptor in different conformation thereby transducing distinct downstream signals. Clearly, the N-terminal and TM1 region near the extracellular domain of glucagon-like peptide-1 receptor play an important role in controlling peptide-mediated biased agonism. These findings provide molecular insights into the initiation of receptor activation and new direction in drug design by maximizing the physiological benefit of biased agonism.
这项研究得到澳洲国家健康与医学研究委员会、国家自然科学基金、中国科学院战略性先导科技专项和上海市科技发展基金的资助,论文的第一作者雷赛飞曾在中国科学院博士生联合培养项目的支持下赴澳研修。
This work was supported by National Health and Medical Research Council of Australia, the National Natural Science Foundation of China, Strategic Priority Research Program of Chinese Academy of Sciences and Shanghai Science and Technology Development Fund. The first author, Saifei Lei, received the Postgraduate Overseas Study Fellowship from Chinese Academy of Sciences when performing part of her research in Australia.
论文链接:/PDF.zip
Link to the paper:/PDF.zip
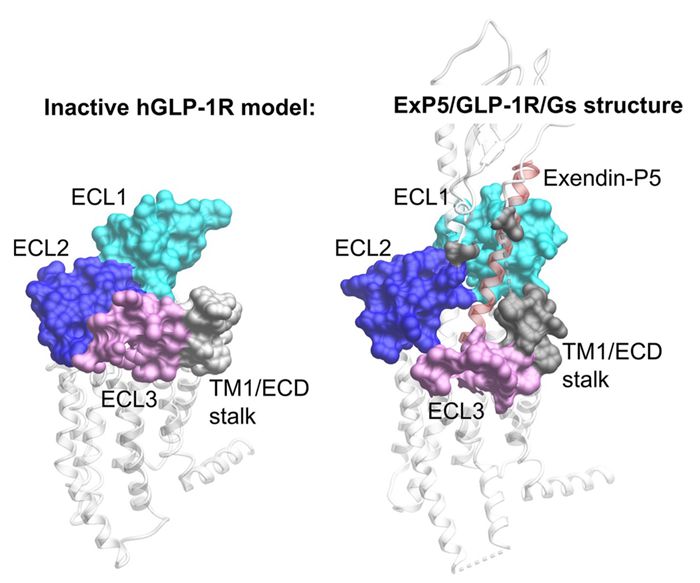
图注:胰高血糖素样肽-1受体未激活状态的结构模型与被Exendin-P5激活的受体与Gs蛋白复合物结构
Inactive glucagon-like peptide-1 receptor model and active structure activated by Exendin-P5 in complex with Gs protein


