间质上皮转化因子(mesenchymal-epithelial transition factor, MET)蛋白是一类跨膜受体,属于络氨酸激酶受体超家族,也是肝素生长因子(hepatocyte growth factor, HGF)的特异性受体。MET信号通路不但促进细胞的增殖、迁移和分化,还在胚胎发育、器官再生及伤口愈合等方面发挥重要作用。MET受体活化能够激活多种下游信号通路,促进肿瘤细胞的增殖、侵袭和转移。MET通路异常与肿瘤耐药、转移风险,不良预后等呈正向相关。
胶质瘤作为最常见的恶性脑肿瘤具有复发率高、死亡率高和预后不良的特点。MET信号过度活化与胶质瘤预后密切相关。研究发现,29%的高级别胶质瘤病人具有MET过度活化特征,且这些病人的中位生存期较MET低表达或不表达病人平均缩短2.6个月。因此,特异性阻断MET/HGF信号通路为胶质瘤向治疗提供新的路径。抗体和小分子抑制剂是两种最主要的MET抑制剂。抗体特异性高,但价格昂贵,且有免疫原性风险;小分子抑制剂具有良好的靶向治疗疗效,但长期使用易产生耐药性。发展新类型的MET抑制剂,不但可以作为抗体和小分子抑制剂外的另一种选择,在降低治疗成本、提高治疗效果,克服耐药性等方面有重要的临床意义。
上海交通大学附属第九人民医院陶晓峰教授课题组与复旦大学药学院李聪教授课题组联合在Nano Letters杂志在线发表了题为“Peptide-Functionalized Nanoinhibitor Restrains Brain Tumor Growth by Abrogating MET Signaling”的研究论文,构建一类标记有多个MET靶向多肽的树枝状高分子纳米抑制剂,其能够有效阻止MET受体的二聚化及其下游信号通路活化从而抑制脑胶质瘤的生长。
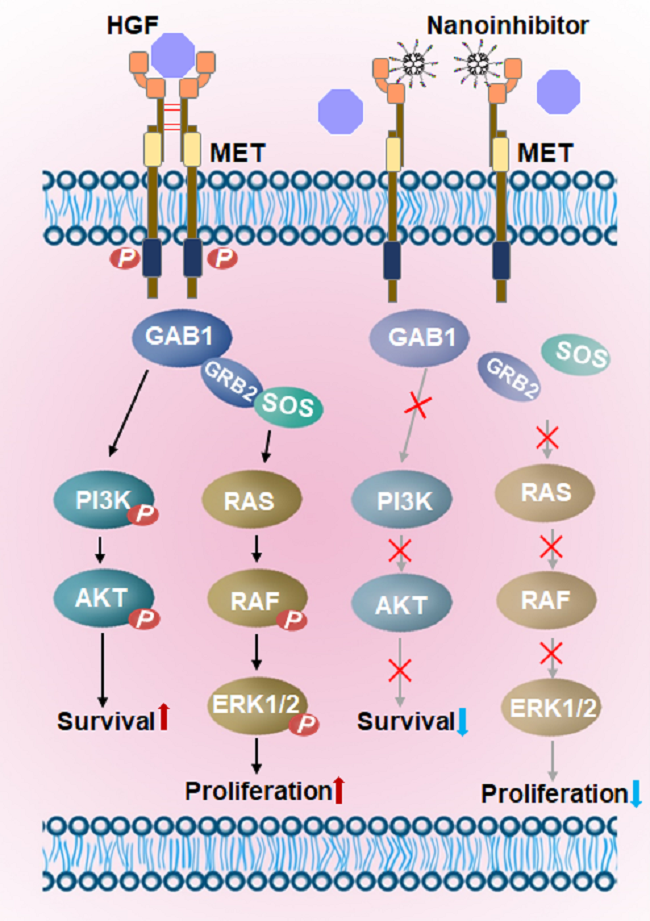
图1. 纳米抑制剂阻止MET受体的二聚化及下游多种信号通路活化
多肽具有高受体亲和力、良好的生物相容性、低免疫原性和可规模生产等优点。但其循环时间短,易被降解等原因在靶向治疗方面的应用受到限制。cMBP是MET受体特异性靶向多肽,与HGF竞争结合MET位点,可以阻止MET受体的二聚化,抑制其下游信号通路的激活。本工作将多条cMBP连接于树枝状高分子得到的纳米抑制剂具有多位点效应,与游离cMBP相比,纳米抑制剂Den-cMBP5和Den-cMBP10对MET受体的结合常数分别提高了32倍和332倍。
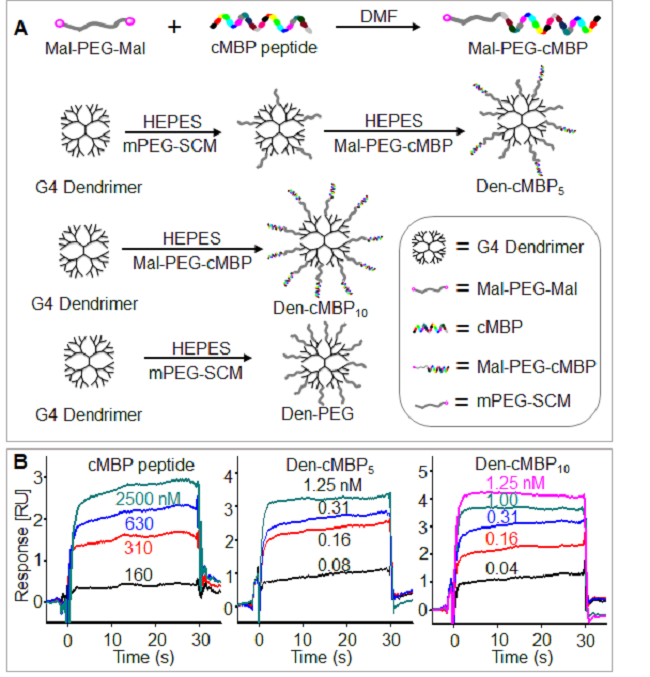
图2. (A) 纳米抑制剂及对照探针的合成。(B) 表面等离子共振测量游离多肽和纳米抑制剂与MET蛋白的结合常数。
相对于正常大鼠星形胶质细胞(RA)和永生化口腔上皮细胞(HIOEC),人脑胶质瘤细胞(U87MG)中MET、AKT、ERK1/2及其磷酸化对应体pMET、pAKT、pERK1/2表达水平均显著提高。病人脑胶质瘤和U87MG原位移植瘤切片均显示pMET在胶质瘤边缘,特别是肿瘤血管腔侧高表达,而在正常脑组织表达量很低。
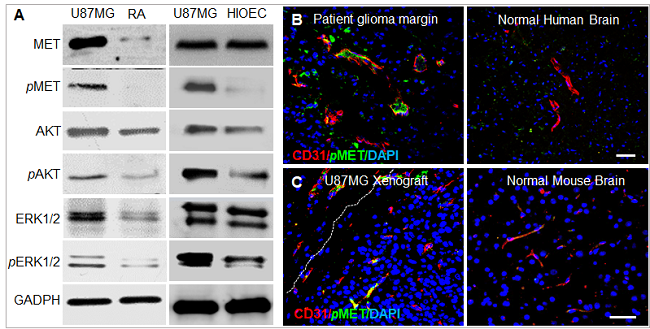
图3. (A) 免疫印迹分析显示MET,pMET及其下游信号通路蛋白在胶质瘤U87MG细胞的表达水平明显高于正常星形胶质RA细胞和正常口腔上皮HIOEC细胞。免疫荧光染色显示pMET在脑胶质瘤病人(B)和U87MG原位移植瘤(C)的表达量明显高于正常脑组织。
与游离cMBP多肽相比,纳米抑制剂Den-cMBP10对U87MG肿瘤细胞呈浓度依赖性抑制。免疫印迹分析结果证明Den-cMBP10能够以浓度依赖性方式有效抑制MET及其下游各个关键信号通路蛋白的磷酸化。
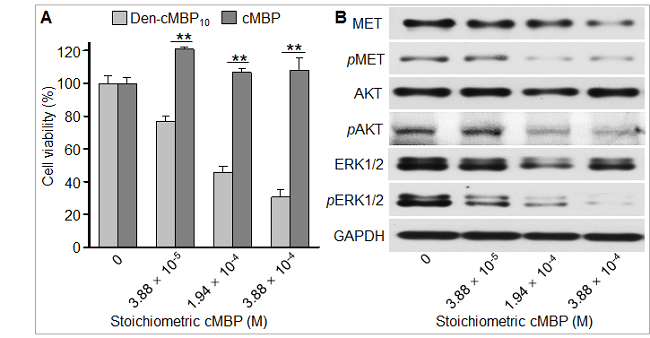
图3. 纳米抑制剂Den-cMBP10抑制MET受体及其下游信号蛋白的磷酸化阻止肿瘤细胞生长。
活体磁共振成像表明,与cMBP多肽相比,Den-cMBP10更好抑制U87MG原位移植瘤的生长,延长荷瘤小鼠的生存期,其抗肿瘤效果与正在进行临床实验的小分子MET抑制剂PF-04217903相当。移植瘤切片的免疫荧光染色显示Den-cMBP10治疗显著降低肿瘤组织pMET的表达量,免疫印迹结果也显示Den-cMBP10治疗降低了小鼠肿瘤MET及其下游信号蛋白的磷酸化。

图4. (A) U87MG原位移植瘤小鼠经治疗后的T2加权磁共振成像。(B) Den-cMBP10有效降低U87MG原位移植瘤中pMET的表达量。(C) Den-cMBP10治疗降低移植瘤中MET及其下游信号蛋白的磷酸化。
基于多肽构建的纳米抑制剂Den-cMBP10通过与HGF竞争MET细胞外结合位点,有效抑制MET信号通路的活化,实现与小分子抑制剂相当的抗肿瘤作用,有望成为抗体和小分子抑制剂外的另一种新类型的MET抑制剂并克服靶向治疗耐药性问题。
上海市第九人民医院吴颖为博士、樊奇博士和复旦大学药学院博士研究生曾峰为该论文的共同第一作者,上海市第九人民医院陶晓峰教授和复旦大学药学院李聪教授为论文的共同通讯作者。美国俄亥俄州立大学Wexner医学中心Karen Briley-Saebo教授参与该项工作。该研究获得国家自然基金委,上海市卫生和计划生育委员会基金会和复旦-中科院上海药物所融合基金的支持。
原文链接:https://pubs.acs.org/doi/full/10.1021/acs.nanolett.8b01879
DOI: 10.1021/acs.nanolett.8b01879
Abstract: Malignant gliomas are the most common primary brain tumors and are associated with aggressive growth, high morbidity, and mortality. Aberrant mesenchymal-epithelial transition factor (MET) activation occurs in approximately 30% of glioma patients and correlates with poor prognosis, elevated invasion, and increased drug resistance. Therefore, MET has emerged as an attractive target for glioma therapy. In this study, we developed a novel nanoinhibitor by conjugating MET-targeting cMBP peptides on the G4 dendrimer. Compared to the binding affinity of the free peptide (KD = 3.96 × 10-7 M), the binding affinity of the nanoinhibitor to MET increased three orders of magnitude to 1.32 × 10-10 M. This nanoinhibitor efficiently reduced the proliferation and invasion of human glioblastoma U87MG cells in vitro by blocking MET signaling with remarkably attenuated levels of phosphorylated MET (pMET) and its downstream signaling proteins, such as pAKT and pERK1/2. Although no obvious therapeutic effect was observed after treatment with free cBMP peptide, in vivo T2-weighted magnetic resonance imaging (MRI) showed a significant delay in tumor growth after intravenous injection of the nanoinhibitor. The medium survival in mouse models was extended by 59%, which is similar to the effects of PF-04217903, a small molecule MET inhibitor currently in clinical trials. Immunoblotting studies of tumor homogenate verified that the nanoinhibitor restrained glioma growth by blocking MET downstream signaling. pMET and its downstream proteins pAKT and pERK1/2, which are involved in the survival and invasion of cancer cells, decreased in the nanoinhibitor-treated group by 44.2%, 62.2%, and 32.3%, respectively, compared with those in the control group. In summary, we developed a peptide-functionalized MET nanoinhibitor that showed extremely high binding affinity to MET and effectively inhibited glioma growth by blocking MET downstream signaling. To the best of our knowledge, this is the first report of therapeutic inhibition of glioma growth by blocking MET signaling with a novel nanoinhibitor. Compared to antibodies and chemical inhibitors in clinical trials, the nanoinhibitor blocks MET signaling and provides a new approach for the treatment of glioma with the advantages of high efficiency, affordability, and most importantly, potentially reduced drug resistance.