美国化学会应用材料与界面杂志(ACS Applied Materials & Interfaces)最近报道了复旦大学药学院和浙江大学医学院附属第二医院联合研究成果:用于导航微小原发性肝肿瘤手术的多模态探针。该探针不但能够在术前通过磁共振成像(MRI)定位微小原发性肝肿瘤(直径1-4 mm),还可以在手术中通过近红外荧光成像引导小微肿瘤的切除。该成果有望实现原发性肝肿瘤的早期发现和手术预后。
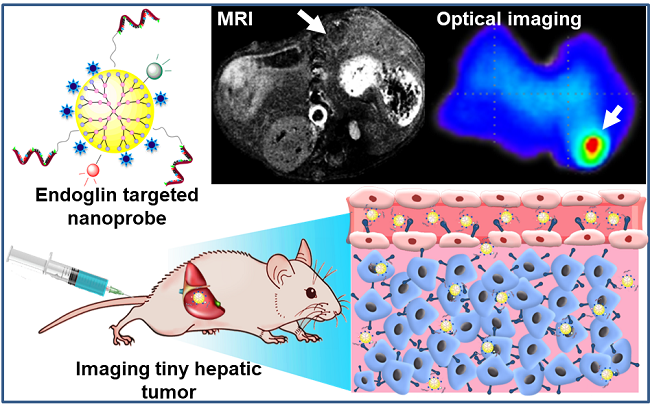
图1. 目标探针通过靶向肿瘤边缘新生血管内皮细胞和肿瘤细胞高表达Endoglin受体实现肿瘤的高信噪比示踪。
原发性肝癌是目前导致癌症相关死亡的第二大原因,而手术切除是原发性肝癌治疗的主要手段。早期接受手术患者在及时切除或移植后可获得高达70%的5年生存率,但仅适用于姑息性治疗的晚期肝癌患者的中位生存期小于1年。然而,原发性肝癌的早期发现是一个巨大的挑战。对于直径小于1.0 cm的微小原发性肝癌,计算机断层扫描(CT)的诊断灵敏度通常低至10-33%,MRI的诊断灵敏度低至29-43%。即使使用了增强造影剂,由于缺乏肿瘤靶向特异性,微小原发性肝癌的诊断率依然较低。考虑到早期接受干预的肝肿瘤患者预后的显著性改善,准确诊断直径小于1.0 cm原发性肝癌对于提高疗效至关重要。
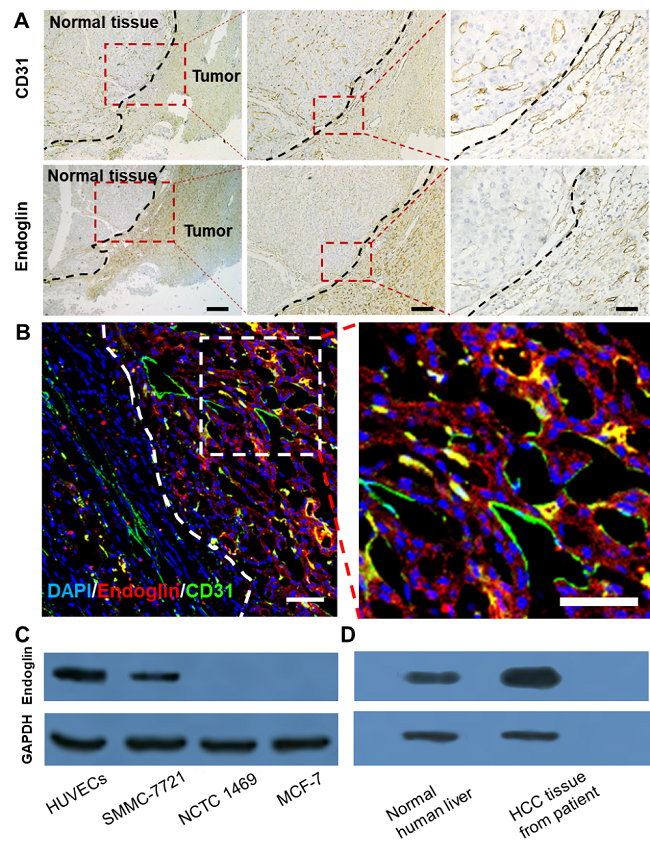
图2. 病人原发性肝癌组织切片H&E染色和免疫荧光染色均显示Endoglin受体在肿瘤边界的表达水平明显高于肿瘤中心和周围正常肝组织。
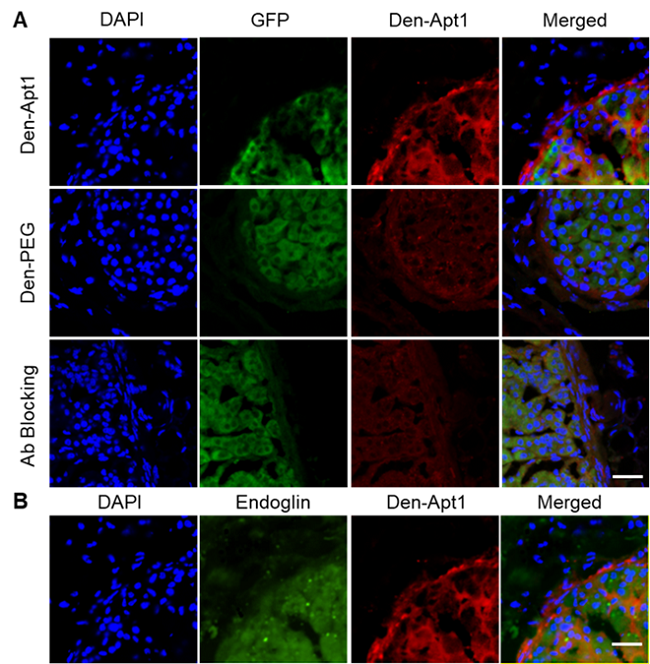
图3. 免疫荧光切片证实,探针(红色)聚集在原发性肝肿瘤模型的边缘区域。它和endoglin受体(B图绿色)的共定位证实了探针的靶向性。
浙江大学医学院附属第二医院消化内科主治医师严蕙蕙博士、复旦大学药学院博士研究生高西辉和硕士研究生张云飞为该论文并列第一作者,严蕙蕙博士、李聪教授和浙江大学医学院附属第二医院消化内科主任医师杜勤为论文的共同通讯作者。李聪教授认为:这是第一个使用适配体作为靶向基团检测微小原发性肝癌的多模态成像探针。它有望在一次注射后同时实现术前和术中微小原发性肝肿瘤的高信噪比示踪。通过手术前定位像和手术中导航像的实时比对还可以避免手术中肝脏位移导致的导航信号失真,进一步提高手术预后。
原文:Imaging Tiny Hepatic Tumor Xenografts via Endoglin-Targeted Paramagnetic/Optical Nanoprobe. DOI:10.1021/acsami.8b02648
https://pubs.acs.org/doi/full/10.1021/acsami.8b02648
Abstract: Surgery is the mainstay for treating hepatocellular carcinoma (HCC). However, it is a great challenge for surgeons to identify HCC in its early developmental stage. The diagnostic sensitivity for a tiny HCC with a diameter less than 1.0 cm is usually as low as 10?33% for computed tomography (CT) and 29?43% for magnetic resonance imaging (MRI). Although MRI is the preferred imaging modality for detecting HCC, with its unparalleled spatial resolution for soft tissue, the commercially available contrast agent, such as Gd3+-DTPA, cannot accurately define HCC because of its short circulation lifetime and lack of tumor-targeting specificity. Endoglin (CD105), a type I membrane glycoprotein, is highly expressed both in HCC cells and in the endothelial cells of neovasculature, which are abundant at the tumor periphery. In this work, a novel single-stranded DNA oligonucleotide-based aptamer was screened by systematic evolution of ligands in an exponential enrichment assay and showed a high binding affinity (KD = 98 pmol/L) to endoglin. Conjugating the aptamers and imaging reporters on a G5 dendrimer created an HCC-targeting nanoprobe that allowed the successful visualization of orthotopic HCC xenografts with diameters as small as 1?4 mm. Significantly, the invasive tumor margin was clearly delineated, with a tumor to normal ratio of 2.7 by near-infrared (NIR) fluorescence imaging and 2.1 by T1-weighted MRI. This multimodal nanoprobe holds promise not only for noninvasively defining tiny HCC by preoperative MRI but also for guiding tumor excision via intraoperative NIR fluorescence imaging, which will probably gain benefit for the patient’s therapeutic response and improve the survival rate.