脑胶质瘤是颅内最常见的原发性恶性肿瘤。神经外科手术是目前胶质瘤主要的治疗手段,且手术切除程度与临床预后呈正相关。胶质瘤浸润性生长导致其与正常脑组织缺乏明显边界,神经外科医生往往根据经验判断手术切除程度。保守切除残留的微小病灶会导致肿瘤早期复发,而激进切除则有可能破坏肿瘤周围的重要功能区,造成失语、瘫痪等严重后遗症。
3月15日,在线发表于《Advanced Materials》杂志上的一项研究中(Guiding Brain-Tumor Surgery via Blood–Brain-Barrier-Permeable Gold Nanoprobes with Acid-Triggered MRI/SERRS Signals),复旦大学药学院李聪教授的研究团队报道了一种对肿瘤酸性环境具有双模态信号响应的探针用于引导胶质瘤的手术切除。
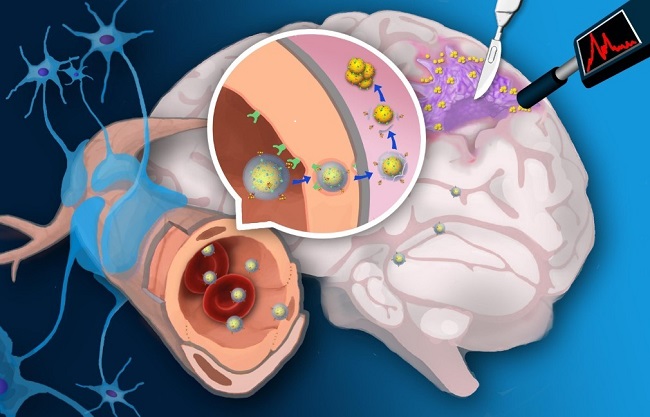
pH双模态响应探针引导胶质瘤切除示意图
该研究第一作者高西辉博士介绍:“该类基于金纳米粒探针通过受体介导转胞吞作用跨越血脑屏障。在肿瘤酸性环境中特异性自组装形成三维球状聚集体并伴随T1加权磁共振信号和表面增强拉曼信号同时增强。高信噪比磁共振成像用于手术前胶质瘤准确定位,而具有超高灵敏(10-12-10-15 M)的表面增强拉曼信号用于手术中引导肿瘤切除”。李聪教授认为:“该类探针的创新性在于:(1)酸性环境是几乎所有固体肿瘤普遍特征,pH响应探针将较少受到肿瘤异质性影响,可实现不同类型胶质瘤普适性示踪和导航;(2)胶质瘤浸润区域血脑屏障结构相对完整。跨血脑屏障探针能够提高对胶质瘤边缘的示踪信噪比和准确率”。
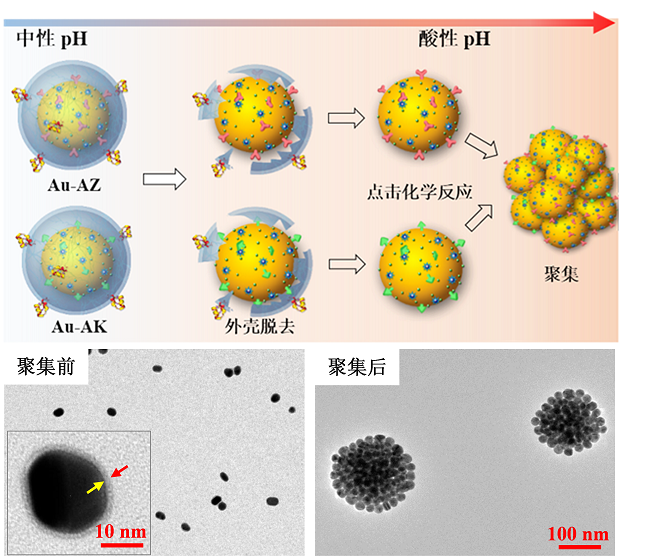
探针在肿瘤酸性环境中特异性聚集示意图和透射电镜图
该研究作者复旦大学附属华山医院神经外科毛颖教授认为:该类双模态影像探针能够实现手术前肿瘤磁共振定位像和手术中拉曼导航像的实时比对,若能应用于临床,则有望克服开颅手术过程中脑移位造成的导航信号偏倚,对提高胶质瘤浸润部位切除率并避免损伤邻近重要脑功能区均有积极临床意义。

探针引导胶质瘤手术切除。(A)手持式拉曼成像仪引导胶质瘤切除示意图;(B)手术过程中探针拉曼信号;(C)H&E组织染色证实探针准确示踪胶质瘤边界。
Title: Guiding Brain-Tumor Surgery via Blood–Brain-Barrier-Permeable Gold Nanoprobes with Acid-Triggered MRI/SERRS Signals. DOI: 10.1002/adma.201603917
Abstract: Surgical resection is a mainstay in the treatment of malignant brain tumors. Surgeons, however, face great challenges in distinguishing tumor margins due to their infiltrated nature. Here, a pair of gold nanoprobes that enter a brain tumor by crossing the blood–brain barrier is developed. The acidic tumor environment triggers their assembly with the concomitant activation of both magnetic resonance (MR) and surface-enhanced resonance Raman spectroscopy (SERRS) signals. While the bulky aggregates continuously trap into the tumor interstitium, the intact nanoprobes in normal brain tissue can be transported back into the blood stream in a timely manner. Experimental results show that physiological acidity triggers nanoparticle assembly by forming 3D spherical nanoclusters with remarkable MR and SERRS signal enhancements. The nanoprobes not only preoperatively define orthotopic glioblastoma xenografts by magnetic resonance imaging (MRI) with high sensitivity and durability in vivo, but also intraoperatively guide tumor excision with the assistance of a handheld Raman scanner. Microscopy studies verify the precisely demarcated tumor margin marked by the assembled nanoprobes. Taking advantage of the nanoprobes' rapid excretion rate and the extracellular acidification as a hallmark of solid tumors, these nanoprobes are promising in improving brain-tumor surgical outcome with high specificity, safety, and universality.
http://onlinelibrary.wiley.com/doi/10.1002/adma.201603917/abstract