癌细胞浸润性生长往往导致恶性肿瘤浸润边缘难以区分,为手术切除带来巨大挑战。由于肿瘤病灶切除完整程度直接关系到手术预后,能够引导肿瘤精准切除的探针对提高手术疗效具有重要的临床意义。
肿瘤细胞高浸润、高侵袭和高转移特征导致其溶酶体内液具有更强的酸性(pHlys 3.8-4.7)。同时,近红外荧光基团凭借其高灵敏度和高组织穿透性在活体光学成像及手术导航中均具有良好的应用前景。李聪课题组奚瑞、张静烨同学设计了五种基于七甲川花箐染料的pH可逆响应近红外荧光探针。通过引入不同取代基,实现了探针pKa值的精准调控(ΔpH = 0.1 pH),其中pKa 4.0的Asp2探针通过对肿瘤细胞溶酶体pH特征响应实现癌细胞高选择性示踪。该探针荧光信号在血液、细胞外液,正常细胞溶酶体环境中均处于淬灭状态,只在癌细胞溶酶体环境中开启。该类型探针对包括脑胶质瘤在内浸润性肿瘤的精准切除提高崭新的工具。
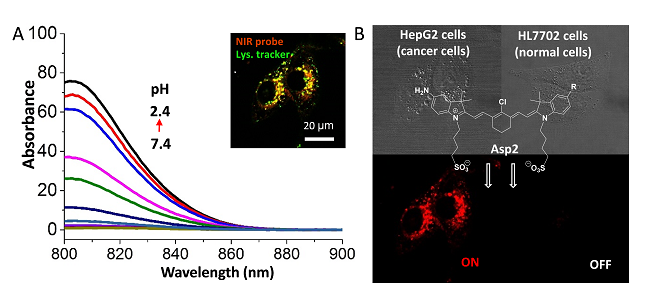
(A) 溶酶体pH敏感近红外荧光探针发射图谱;(B) pH敏感荧光探针选择性示踪肿瘤细胞
该研究成果最近发表于《RSC Advances》杂志。
http://pubs.rsc.org/en/content/articlelanding/2016/ra/c6ra12381c
Title:
Near-infrared asymmetrical heptamethine cyanines specifically imaging cancer cells by sensing their acidic lysosomal lumen
Abstract:
Tumor cytoreductive surgery faces great challenges to completely remove malignant residues due to the indistinct margin between the normal and neoplastic tissues. Intra-operative delineation of a tumor invasive margin will greatly improve the prognosis of the surgery. Near-infrared (NIR) fluorescence imaging shows advantages in delineating a tumor margin due to its high sensitivity and deep tissue penetration depth. Therefore, it is important to develop NIR fluorophores with high specificity and sensitivity. Cancer cells demonstrate more acidic lysosomal lumen (pH 3.8–4.7) than that of normal cells (pH 4.5–6.0), which facilitates their invasion, migration and metastasis. In this work, we developed five asymmetrical heptamethine cyanine (Hcyanine) based NIR fluorophores, in which a primary amine as a proton sensitizing group and another functional group as an electron density modulator were labeled on the Hcyanine respectively. While these fluorophores remained silent under a neutral environment, their fluorescence quantum yields increased 18–54 times upon the acidification from pH 7.4 to 2.54. Modulating the electron density on the fluorophores could fine tune their pKa (ΔpH = 0.1 pH units) to fit the lysosomal pH (pHlys) in cancer cells. AsP2 with a pKa of 4.0 visualized cancer cells, especially the invasive cancer cells with high selectivity. Even though AsP2 remained silent in the normal cells, its activation in the lysosomes after cytoplasmic acidification verified the feasibility to specifically detect tumor by distinguishing the pHlys discrepancy between the normal and cancer cells. Considering the up-regulated lysosomal acidity is a universal characteristic of cancer cells, pHlys responsive fluorophores are promising to accurately delineate tumor margin and guide intra-operative tumor resection with high sensitivity and universality.