内皮细胞间“紧密连接”是维持血脑屏障(BBB)功能的关键。目前临床使用高渗剂如甘露醇通过提高毛细血管两侧的渗透压“挤破”紧密连接从而提高BBB通透性。但该方法对BBB不可控开启往往引起脑水肿和神经损伤等副作用。因此,构建高效、安全调控BBB通透性新策略对提高中枢神经系统疾病疗效具有重要临床意义。
复旦大学李聪课题组高西辉同学制备了一种基于树枝状高分子的纳米激动剂。该纳米激动剂可特异激活脑毛细血管内皮细胞上的腺苷A2A受体从而引起内皮细胞收缩、紧密连接暂时性开启。活体磁共振成像证实纳米激动剂能够显著提高正常小鼠脑血管通透性及模型药物入脑效率。透射电镜直接观察到紧密连接的开启及可逆恢复。免疫荧光显微成像表明血管基膜、周细胞、胶质细胞周足和内皮细胞等BBB主要结构未有损伤。组织切片也未发现脑水肿、细胞凋亡、神经损伤等副作用。纳米激动剂的优势在于:1. 通过内源性机制可逆调控血脑屏障通透性,安全性好;2. 通过调控激动基团数目调节BBB开启效率和时间窗口,为不同类型药物个性化入脑提供新的途径。
该研究成果最近发表于《Journal of Cerebral Blood Flow & Metabolism》杂志。
http://jcb.sagepub.com/content/early/2016/06/23/0271678X16656198.abstract
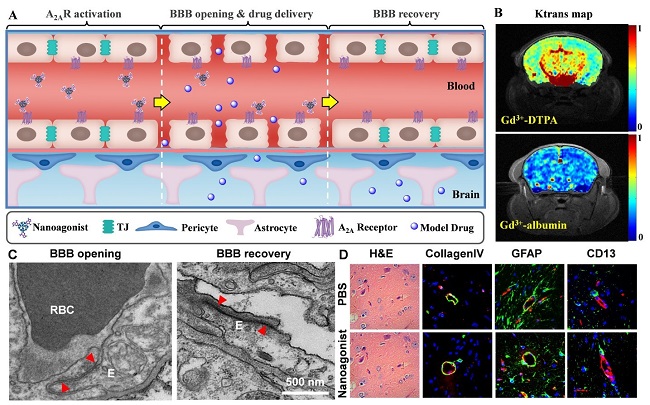
A)纳米激动剂可逆开启BBB示意图;(B)动态增强磁共振成像(DCE-MRI)显示纳米激动剂提高BBB通透性;(C)透射电镜显示注射纳米激动剂后紧密连接的开启及可逆恢复;(D)组织染色表明血管基膜、胶质细胞周足、周细胞和内皮细胞等BBB主要组分未有损伤,也未发现脑水肿、神经凋亡等副作用。
原文标题: Nanoagonist-mediated endothelial tight junction opening: A strategy for safely increasing brain drug delivery in mice
Abstract: Even though opening endothelial tight junctions is an efficient way to up-regulate brain drug delivery, the extravasation of blood-borne components from the compromised tight junctions can result in adverse consequences such as edema and neuronal injuries. In this work, we developed a nanoagonist that temporarily opened tight junctions by signaling adenosine 2A receptor, a type of G protein-coupled receptor expressed on brain capillary endothelial cells. Magnetic resonance imaging demonstrated remarkable blood–brain barrier permeability enhancements and significantly increased brain uptakes of both small molecular and macromolecular paramagnetic agents after nanoagonist administration. Gamma ray imaging and transmission electron microscope observed tight junction opening followed by spontaneous recovery after nanoagonist treatment. Immunofluorescence staining showed the unspoiled basal membrane, pericytes and astrocyte endfeet that enwrapped the vascular endothelium. Importantly, edema, apoptosis and neuronal injuries observed after hypertonic agent mediated tight junction-opening were not observed after nanoagonist intervention. The uncompromised neurovascular units may prevent the leakage of blood-borne constituents into brain parenchyma and accelerate tight junction recovery. Considering blood–brain barrier impermeability is a major obstacle in the treatment of central nervous system diseases, nanoagonist-mediated tight junction opening provides a promising strategy to enhance brain drug delivery with minimized adverse effects.