恶性肿瘤细胞往往呈浸润性生长,与正常组织呈犬牙交错状。尽可能切除零星分布的肿瘤病灶,同时避免对邻近正常组织过度切除造成负面影响是外科医生面临的巨大挑战。此外,生长在大血管、不可再生神经或是具有重要功能器官周围微小肿瘤,往往难以通过手术刀直接切除。因此,能够对微小病灶准确示踪并及时消融的诊疗一体化探针具有重要的临床意义。
与正常细胞溶酶体(pHlys 4.5-6.0)相比,肿瘤细胞高浸润、高侵袭和高转移特征导致其溶酶体内液往往具有更强的酸性(pHlys 3.8-4.7)。根据此特征,复旦大学药学院李聪教授课题组张静烨同学制备了一类基于七甲川花箐染料的近红外荧光/光热pH逆向双响应探针。该探针在生理酸性条件下,所吸收能量主要以近红外荧光形式释放;然而在中性或弱碱性环境中,能量主要以热的形式释放。通过精准调控其pKa值(4.6),该探针荧光信号只在癌细胞溶酶体酸性环境中开启,实现癌细胞的选择性示踪。同时,该探针还可以进入癌细胞线粒体,其弱碱性环境使探针光热效应最大化,导致线粒体膜通透性改变,诱导癌细胞凋亡/坏死。溶酶体pH异常是肿瘤细胞的普遍特征,该类诊疗一体化探针可较少受到肿瘤基因类型和表型影响,对影像引导的术中肿瘤切除和具有重要意义。
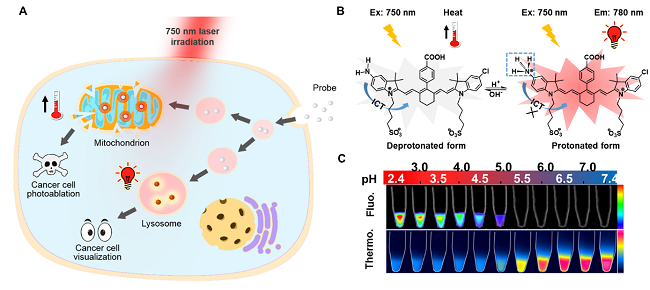
(A)探针选择性示踪并光热消除肿瘤细胞;(B&C)探针pH逆向双响应近红外荧光和光热信号
该研究成果最近发表于《Chemical Science》杂志。相关动物实验正在进行中。
http://pubs.rsc.org/en/content/articlelanding/2014/SC/C6SC00221H#!divAbstract
原文标题:
Selective imaging and cancer cell death via pH switchable near-infrared fluorescence and photothermal effects
摘要:
Accurately locating and eradicating sporadically distributed cancer cells whilst minimizing damage to adjacent normal tissues is vital in image-guided tumor ablation. In this work, we developed four heptamethine cyanine based theranostic probes, IR1–4, that demonstrated unique pH switchable near-infrared (NIR) fluorescence and photothermal efficiency. While their fluorescence quantum yields increased up to 1020-fold upon acidification from pH 7.4 to 2.4, their photothermal efficiencies decreased up to 7.1-fold concomitantly. Theoretical calculations showed that protonation of the probes in acidic environment increased the orbital energy gaps and reduced the intramolecular charge transfer efficiency, resulting in the conversion of absorbed light energy to NIR fluorescence instead of hyperthermia. Substitutions at the terminal indole of the probes fine-tuned their pKafluo values to a narrow physiological pH range of 4.0–5.3. IR2, with pKafluo of 4.6, not only specifically illuminated cancer cells by sensing their more acidic lysosomal lumen, but also selectively ablated cancer cells via its maximized photothermal effects in the alkaline mitochondrial matrix. As far as we are aware, these probes not only offer the highest physiological acidity triggered NIR fluorescence enhancement as small molecules, but are also the first to specifically visualize and eradicate cancer cells by sensing their altered pHs in cellular organelles. Considering that the disordered pH in organelle lumen is a common characteristic of cancer cells, these theranostic probes hold the promise to be applied in image-guided tumor ablation over a wide range of tumor subtypes.